Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D02FCU
|
|||
| Former ID |
DCL001214
|
|||
| Drug Name |
MIV-210
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Hepatitis B virus infection [ICD-11: 1E51.0; ICD-10: B18.1] | Phase 2 | [1] | |
| Company |
Medivir; Daewoong
|
|||
| Structure |
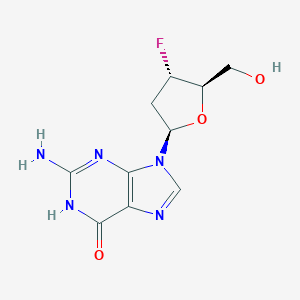 |
Download2D MOL |
||
| Formula |
C10H12FN5O3
|
|||
| Canonical SMILES |
C1C(C(OC1N2C=NC3=C2N=C(NC3=O)N)CO)F
|
|||
| InChI |
1S/C10H12FN5O3/c11-4-1-6(19-5(4)2-17)16-3-13-7-8(16)14-10(12)15-9(7)18/h3-6,17H,1-2H2,(H3,12,14,15,18)/t4-,5+,6+/m0/s1
|
|||
| InChIKey |
RTJUXLYUUDBAJN-KVQBGUIXSA-N
|
|||
| CAS Number |
CAS 92562-88-4
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Hepatitis B virus Reverse transcriptase (HBV RT) | Target Info | Modulator | [2] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Clinical pipeline report, company report or official report of Medivir (2011). | |||
| REF 2 | Emerging antivirals for the treatment of hepatitis B | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

