Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D01SGK
|
|||
| Former ID |
DIB006208
|
|||
| Drug Name |
Opicapone
|
|||
| Synonyms |
BIA-9-1067
Click to Show/Hide
|
|||
| Indication | Parkinson disease [ICD-11: 8A00.0; ICD-10: F02.3, G20; ICD-9: 332] | Approved | [1] | |
| Company |
Portela & Ca SA
|
|||
| Structure |
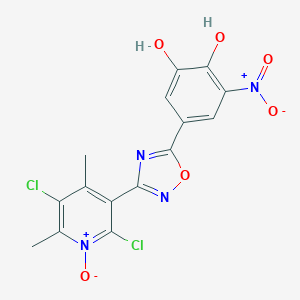 |
Download2D MOL |
||
| Formula |
C15H10Cl2N4O6
|
|||
| Canonical SMILES |
CC1=C(C(=[N+](C(=C1Cl)C)[O-])Cl)C2=NOC(=N2)C3=CC(=C(C(=C3)O)O)[N+](=O)[O-]
|
|||
| InChI |
1S/C15H10Cl2N4O6/c1-5-10(13(17)20(24)6(2)11(5)16)14-18-15(27-19-14)7-3-8(21(25)26)12(23)9(22)4-7/h3-4,22-23H,1-2H3
|
|||
| InChIKey |
ASOADIZOVZTJSR-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 923287-50-7
|
|||
| PubChem Compound ID | ||||
| ChEBI ID |
CHEBI:134699
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Catechol-O-methyl-transferase (COMT) | Target Info | Inhibitor | [1] |
| BioCyc | L-dopa degradation | |||
| Dopamine degradation | ||||
| Noradrenaline and adrenaline degradation | ||||
| KEGG Pathway | Steroid hormone biosynthesis | |||
| Tyrosine metabolism | ||||
| Metabolic pathways | ||||
| Dopaminergic synapse | ||||
| Panther Pathway | Adrenaline and noradrenaline biosynthesis | |||
| Dopamine receptor mediated signaling pathway | ||||
| Pathwhiz Pathway | Tyrosine Metabolism | |||
| WikiPathways | Methylation Pathways | |||
| Metapathway biotransformation | ||||
| Estrogen metabolism | ||||
| Biogenic Amine Synthesis | ||||
| Dopamine metabolism | ||||
| Phase II conjugation | ||||
| Neurotransmitter Clearance In The Synaptic Cleft | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health Human Services. 2020 | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

