Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D01KOA
|
|||
| Drug Name |
Selpercatinib
|
|||
| Synonyms |
MFOVQWYFURMVKU-IWAAJCSBSA-N; 2222755-14-6; LOXO292; ARRY-192; LOXO 292; EX-A2636
Click to Show/Hide
|
|||
| Indication | Non-small-cell lung cancer [ICD-11: 2C25.Y; ICD-9: 162] | Approved | [1] | |
| Thyroid cancer [ICD-11: 2D10; ICD-10: C73; ICD-9: 193] | Approved | [1] | ||
| Colon cancer [ICD-11: 2B90.Z] | Phase 1/2 | [2] | ||
| Company |
Loxo Oncology Stamford, CT
|
|||
| Structure |
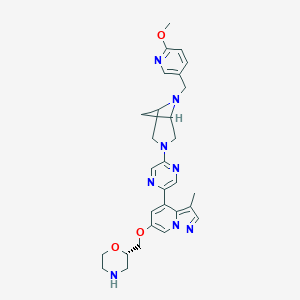 |
Download2D MOL |
||
| Formula |
C29H34N8O3
|
|||
| Canonical SMILES |
CC1=C2C(=CC(=CN2N=C1)OCC3CNCCO3)C4=CN=C(C=N4)N5CC6CC(C5)N6CC7=CN=C(C=C7)OC
|
|||
| InChI |
1S/C29H34N8O3/c1-19-9-34-37-17-23(40-18-24-11-30-5-6-39-24)8-25(29(19)37)26-12-32-27(13-31-26)35-15-21-7-22(16-35)36(21)14-20-3-4-28(38-2)33-10-20/h3-4,8-10,12-13,17,21-22,24,30H,5-7,11,14-16,18H2,1-2H3/t21?,22?,24-/m0/s1
|
|||
| InChIKey |
MFOVQWYFURMVKU-IWAAJCSBSA-N
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Proto-oncogene c-Ret (RET) | Target Info | Inhibitor | [1] |
| KEGG Pathway | Endocytosis | |||
| Pathways in cancer | ||||
| Thyroid cancer | ||||
| Central carbon metabolism in cancer | ||||
| Pathway Interaction Database | Signaling events regulated by Ret tyrosine kinase | |||
| Posttranslational regulation of adherens junction stability and dissassembly | ||||
| WikiPathways | SIDS Susceptibility Pathways | |||
| Dopaminergic Neurogenesis | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health Human Services. 2020 | |||
| REF 2 | ClinicalTrials.gov (NCT03157128) A Study of LOXO-292 in Participants With Advanced Solid Tumors, RET Fusion-Positive Solid Tumors, and Medullary Thyroid Cancer (LIBRETTO-001). U.S. National Institutes of Health. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

