Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D00SFY
|
|||
| Former ID |
DIB015772
|
|||
| Drug Name |
BI-44847
|
|||
| Indication | Type-2 diabetes [ICD-11: 5A11; ICD-9: 250] | Phase 1 | [1] | |
| Company |
Ajinomoto Co Inc
|
|||
| Structure |
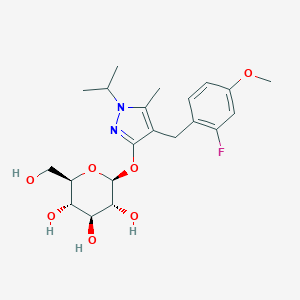 |
Download2D MOL |
||
| Formula |
C21H29FN2O7
|
|||
| Canonical SMILES |
CC1=C(C(=NN1C(C)C)OC2C(C(C(C(O2)CO)O)O)O)CC3=C(C=C(C=C3)OC)F
|
|||
| InChI |
1S/C21H29FN2O7/c1-10(2)24-11(3)14(7-12-5-6-13(29-4)8-15(12)22)20(23-24)31-21-19(28)18(27)17(26)16(9-25)30-21/h5-6,8,10,16-19,21,25-28H,7,9H2,1-4H3/t16-,17-,18+,19-,21+/m1/s1
|
|||
| InChIKey |
AOBLNYCSGWSIPG-YRIDSSQKSA-N
|
|||
| CAS Number |
CAS 421592-30-5
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Sodium/glucose cotransporter 2 (SGLT2) | Target Info | Modulator | [2] |
| Reactome | Hexose transport | |||
| Na+-dependent glucose transporters | ||||
| Inositol transporters | ||||
| WikiPathways | NRF2 pathway | |||
| Nuclear Receptors Meta-Pathway | ||||
| Transport of glucose and other sugars, bile salts and organic acids, metal ions and amine compounds | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT02211937) Relative Oral Bioavailability of BI 44847 as Suspension Compared to Tablet and the Influence of Food Anf of BI 44847 as Solution Compared to Tablet in Healthy Male Volunteers. U.S. National Institutes of Health. | |||
| REF 2 | Clinical potential of sodium-glucose cotransporter 2 inhibitors in the management of type 2 diabetes | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

