Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D00QKI
|
|||
| Former ID |
DCL000980
|
|||
| Drug Name |
SCH-527123
|
|||
| Synonyms |
Navarixin; 473727-83-2; Sch527123; SCH 527123; (R)-2-Hydroxy-N,N-dimethyl-3-((2-((1-(5-methylfuran-2-yl)propyl)amino)-3,4-dioxocyclobut-1-en-1-yl)amino)benzamide; MK-7123; CHEMBL216981; (R)-2-hydroxy-N,N-dimethyl-3-(2-(1-(5-methylfuran-2-yl)propylamino)-3,4-dioxocyclobut-1-enylamino)benzamide; SCHEMBL184744; GTPL8497; KS-00001CQK; CTK8B8735; MolPort-023-331-228; BDBM50200880; MK7123; ANW-61143; ZINC100033051; AKOS016003539; CS-0609; NCGC00390675-01; HY-10198; AX8217127; TC-149888; W-5650; SCH 527123,CAS:473727-83-2; PF-00547659
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Chronic obstructive pulmonary disease [ICD-11: CA22; ICD-10: J44, J44.9] | Phase 2 | [1], [2] | |
| Inflammatory bowel disease [ICD-11: DD72; ICD-10: K52.3] | Phase 2 | [3] | ||
| Ulcerative colitis [ICD-11: DD71] | Phase 2 | [3] | ||
| Solid tumour/cancer [ICD-11: 2A00-2F9Z] | Phase 1 | [4] | ||
| Company |
Pharmacopeia; Schering-Plough
|
|||
| Structure |
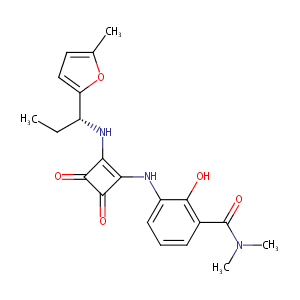 |
Download2D MOL |
||
| Formula |
C21H23N3O5
|
|||
| Canonical SMILES |
CCC(C1=CC=C(O1)C)NC2=C(C(=O)C2=O)NC3=CC=CC(=C3O)C(=O)N(C)C
|
|||
| InChI |
1S/C21H23N3O5/c1-5-13(15-10-9-11(2)29-15)22-16-17(20(27)19(16)26)23-14-8-6-7-12(18(14)25)21(28)24(3)4/h6-10,13,22-23,25H,5H2,1-4H3/t13-/m1/s1
|
|||
| InChIKey |
RXIUEIPPLAFSDF-CYBMUJFWSA-N
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 8497). | |||
| REF 2 | Emerging drugs in chronic obstructive pulmonary disease. Expert Opin Emerg Drugs. 2009 Mar;14(1):181-94. | |||
| REF 3 | Emerging drugs to treat Crohn's disease. Expert Opin Emerg Drugs. 2007 Mar;12(1):49-59. | |||
| REF 4 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 5 | CXCR2 Antagonist MK-7123.A Phase 2 Proof-of-Concept Trial for Chronic Obstructive Pulmonary Disease.Am J Respir Crit Care Med.2015 May 1;191(9):1001-11. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

