Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D00GZY
|
|||
| Former ID |
DNC014897
|
|||
| Drug Name |
AT1001
|
|||
| Synonyms |
Larazotide acetate; Larazotide acetate [USAN]; AT-1001; 881851-50-9; Larazotide acetate (USAN); UNII-FO8S2IW40N; AT 1001; FO8S2IW40N; CHEMBL2103815; NYGCNONRVCGHAT-UFIKZEAMSA-N; HY-106268A; CS-8047; D09352; Glycylglycyl-L-valyl-L-leucyl-L-valyl-L-glutaminyl-L-prolylglycine acetate; Glycine, glycylglycyl-L-valyl-L-leucyl-L-valyl-L-glutaminyl-L-prolyl-, monoacetate
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Autoimmune diabetes [ICD-11: 5A10] | Phase 3 | [1] | |
| Structure |
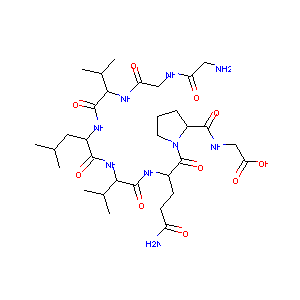 |
Download2D MOL |
||
| Formula |
C34H59N9O12
|
|||
| Canonical SMILES |
CC(C)CC(C(=O)NC(C(C)C)C(=O)NC(CCC(=O)N)C(=O)N1CCCC1C(=O)NCC(=O)O)NC(=O)C(C(C)C)NC(=O)CNC(=O)CN.CC(=O)O
|
|||
| InChI |
1S/C32H55N9O10.C2H4O2/c1-16(2)12-20(38-30(49)26(17(3)4)39-24(44)14-35-23(43)13-33)28(47)40-27(18(5)6)31(50)37-19(9-10-22(34)42)32(51)41-11-7-8-21(41)29(48)36-15-25(45)46;1-2(3)4/h16-21,26-27H,7-15,33H2,1-6H3,(H2,34,42)(H,35,43)(H,36,48)(H,37,50)(H,38,49)(H,39,44)(H,40,47)(H,45,46);1H3,(H,3,4)/t19-,20-,21-,26-,27-;/m0./s1
|
|||
| InChIKey |
NYGCNONRVCGHAT-UFIKZEAMSA-N
|
|||
| CAS Number |
CAS 881851-50-9
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Haptoglobin (HP) | Target Info | Modulator | [2] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT00925301) Study of the Effects of Oral AT1001 (Migalastat Hydrochloride) in Patients With Fabry Disease. U.S. National Institutes of Health. | |||
| REF 2 | Zonulin, regulation of tight junctions, and autoimmune diseases | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

