Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D00GPQ
|
|||
| Former ID |
DNCL002194
|
|||
| Drug Name |
Nirogacestat
|
|||
| Synonyms |
PF-03084014; 1290543-63-3; PF-03084014; PF-3084014; UNII-QZ62892OFJ; 865773-15-5; PF 3084014; PF-03084014 (PF-3084014); QZ62892OFJ; Z-3181; PF03084014; Nirogacestat [USAN]; Nirogacestat (USAN/INN); GTPL7746; SCHEMBL13184754; CHEMBL1770916; EX-A855; DTXSID60235679; MolPort-039-193-852; (2S)-2-[[(2S)-6,8-difluoro-1,2,3,4-tetrahydronaphthalen-2-yl]amino]-N-[1-[1-(2,2-dimethylpropylamino)-2-methylpropan-2-yl]imidazol-4-yl]pentanamide; ZINC38217837; s8018; AKOS030526383; SB16726; DB12005; CS-1689; NCGC00378713-01
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Desmoid tumour [ICD-11: 2F7C] | Approved | [1] | |
| Alzheimer disease [ICD-11: 8A20; ICD-10: G30] | Phase 2 | [2], [3] | ||
| Solid tumour/cancer [ICD-11: 2A00-2F9Z] | Phase 2 | [4] | ||
| Company |
Springworks
|
|||
| Structure |
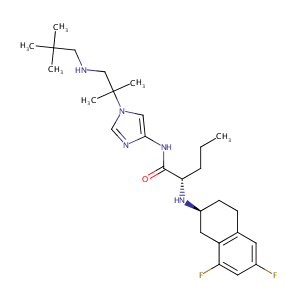 |
Download2D MOL |
||
| Formula |
C27H41F2N5O
|
|||
| Canonical SMILES |
CCCC(C(=O)NC1=CN(C=N1)C(C)(C)CNCC(C)(C)C)NC2CCC3=C(C2)C(=CC(=C3)F)F
|
|||
| InChI |
1S/C27H41F2N5O/c1-7-8-23(32-20-10-9-18-11-19(28)12-22(29)21(18)13-20)25(35)33-24-14-34(17-31-24)27(5,6)16-30-15-26(2,3)4/h11-12,14,17,20,23,30,32H,7-10,13,15-16H2,1-6H3,(H,33,35)/t20-,23-/m0/s1
|
|||
| InChIKey |
VFCRKLWBYMDAED-REWPJTCUSA-N
|
|||
| CAS Number |
CAS 1290543-63-3
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | FDA Approved Drug Products from FDA Official Website. 2023. Application Number: 217677 | |||
| REF 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7746). | |||
| REF 3 | ClinicalTrials.gov (NCT02299635) A Study Evaluating PF-03084014 In Patients With Advanced Breast Cancer With Or Without Notch Alterations. U.S. National Institutes of Health. | |||
| REF 4 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

