Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D00CGH
|
|||
| Former ID |
DIB003221
|
|||
| Drug Name |
RO-23-7553
|
|||
| Synonyms |
BXL-353; ILX-23-7553; (1alpha,3beta,5Z,7E)-9,10-Secocholesta-5,7,10(19),16-tetraen-23-yne-1,3,25-triol; 1alpha,25-Dihydroxy-16,17,23,23,24,24-hexadehydrocholecalciferol; 1alpha,25-Dihydroxy-16,17,23,23,24,24-hexadehydrovitamin D3
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Prostate disease [ICD-11: GA91; ICD-10: N42.9] | Phase 1 | [1] | |
| Structure |
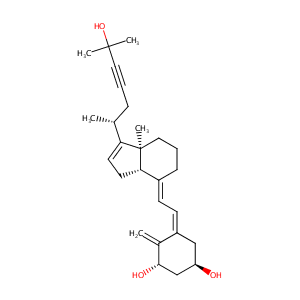 |
Download2D MOL |
||
| Formula |
C27H38O3
|
|||
| Canonical SMILES |
CC(CC#CC(C)(C)O)C1=CCC2C1(CCCC2=CC=C3CC(CC(C3=C)O)O)C
|
|||
| InChI |
1S/C27H38O3/c1-18(8-6-14-26(3,4)30)23-12-13-24-20(9-7-15-27(23,24)5)10-11-21-16-22(28)17-25(29)19(21)2/h10-12,18,22,24-25,28-30H,2,7-9,13,15-17H2,1,3-5H3/b20-10+,21-11-/t18-,22-,24+,25+,27-/m1/s1
|
|||
| InChIKey |
JKFZMIQMKFWJAY-RQJQXFIZSA-N
|
|||
| CAS Number |
CAS 118694-43-2
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Vitamin D3 receptor (VDR) | Target Info | Agonist | [2], [3] |
| KEGG Pathway | Endocrine and other factor-regulated calcium reabsorption | |||
| Mineral absorption | ||||
| Tuberculosis | ||||
| NetPath Pathway | IL4 Signaling Pathway | |||
| Panther Pathway | Vitamin D metabolism and pathway | |||
| Pathway Interaction Database | Regulation of nuclear SMAD2/3 signaling | |||
| Direct p53 effectors | ||||
| RXR and RAR heterodimerization with other nuclear receptor | ||||
| Retinoic acid receptors-mediated signaling | ||||
| Validated transcriptional targets of deltaNp63 isoforms | ||||
| Validated transcriptional targets of TAp63 isoforms | ||||
| Reactome | Nuclear Receptor transcription pathway | |||
| WikiPathways | Ovarian Infertility Genes | |||
| Nuclear Receptors in Lipid Metabolism and Toxicity | ||||
| Nuclear Receptors Meta-Pathway | ||||
| Vitamin D Receptor Pathway | ||||
| Drug Induction of Bile Acid Pathway | ||||
| Nuclear Receptors | ||||
| Vitamin D Metabolism | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT00004926) ILX23-7553 in Treating Patients With Solid Tumors That Have Not Responded to Previous Therapy. U.S. National Institutes of Health. | |||
| REF 2 | Three synthetic vitamin D analogues induce prostate-specific acid phosphatase and prostate-specific antigen while inhibiting the growth of human prostate cancer cells in a vitamin D receptor-dependent fashion. Clin Cancer Res. 1997 Aug;3(8):1331-8. | |||
| REF 3 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

