Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
DSHT18
|
|||
| Drug Name |
Asciminib
|
|||
| Synonyms |
ABL-001; 1492952-76-7; Asciminib free base; ABL001-NX; UNII-L1F3R18W77; L1F3R18W77; NVP-ABL001; (R)-N-(4-(Chlorodifluoromethoxy)phenyl)-6-(3-hydroxypyrrolidin-1-yl)-5-(1H-pyrazol-5-yl)nicotinamide; 1492952-76-7 (free base); N-[4-[chloro(difluoro)methoxy]phenyl]-6-[(3R)-3-hydroxypyrrolidin-1-yl]-5-(1H-pyrazol-5-yl)pyridine-3-carboxamide; Asciminib [USAN]; Asciminib (ABL001); Asciminib (USAN/INN); GTPL8962; CHEMBL4208229; SCHEMBL15388306; TQP0925; EX-A3030; BDBM50459091; NSC789925; s8555; ZINC150275965; CCG-269232; CS-7655; DB12597; NSC-789925; SB18878; BS-15538; HY-104010; D11403; Q27074535; (R)-N-(4-(Chloro difluoromethoxy)phenyl)-6-(3-hydroxypyrrolidin-1-yl)-5-(1H-pyrazol-5-yl)nicotinamide; AY7
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Chronic myeloid leukaemia [ICD-11: 2A20; ICD-10: C92.7; ICD-9: 205.1] | Approved | [1] | |
| Company |
Novartis
|
|||
| Structure |
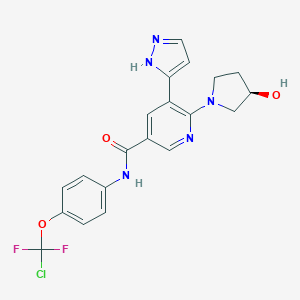 |
Download2D MOL |
||
| Formula |
C20H18ClF2N5O3
|
|||
| Canonical SMILES |
C1CN(CC1O)C2=C(C=C(C=N2)C(=O)NC3=CC=C(C=C3)OC(F)(F)Cl)C4=CC=NN4
|
|||
| InChI |
1S/C20H18ClF2N5O3/c21-20(22,23)31-15-3-1-13(2-4-15)26-19(30)12-9-16(17-5-7-25-27-17)18(24-10-12)28-8-6-14(29)11-28/h1-5,7,9-10,14,29H,6,8,11H2,(H,25,27)(H,26,30)/t14-/m1/s1
|
|||
| InChIKey |
VOVZXURTCKPRDQ-CQSZACIVSA-N
|
|||
| CAS Number |
CAS 1492952-76-7
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Fusion protein Bcr-Abl (Bcr-Abl) | Target Info | Inhibitor | [2] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | FDA Approved Drug Products from FDA Official Website. 2022. Application Number: 215358. | |||
| REF 2 | Asciminib in Chronic Myeloid Leukemia after ABL Kinase Inhibitor Failure. N Engl J Med. 2019 Dec 12;381(24):2315-2326. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

