Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0V8HJ
|
|||
| Former ID |
DNCL001657
|
|||
| Drug Name |
Fostamatinib
|
|||
| Synonyms |
901119-35-5; R788; Tavalisse; UNII-SQ8A3S5101; R-788 Free acid; R 788; R-788; R-935788 Free acid; SQ8A3S5101; R7935788; Fostamatinib [USAN:INN]
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Immune thrombocytopenic purpura [ICD-11: 3B64.13; ICD-10: D69; ICD-9: 287.31] | Approved | [1] | |
| Rheumatoid arthritis [ICD-11: FA20] | Phase 3 | [2], [3] | ||
| Company |
Rigel Pharmaceuticals
|
|||
| Structure |
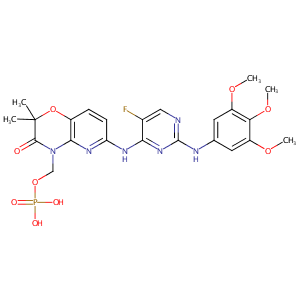 |
Download2D MOL |
||
| Formula |
C23H26FN6O9P
|
|||
| Canonical SMILES |
CC1(C(=O)N(C2=C(O1)C=CC(=N2)NC3=NC(=NC=C3F)NC4=CC(=C(C(=C4)OC)OC)OC)COP(=O)(O)O)C
|
|||
| InChI |
1S/C23H26FN6O9P/c1-23(2)21(31)30(11-38-40(32,33)34)20-14(39-23)6-7-17(28-20)27-19-13(24)10-25-22(29-19)26-12-8-15(35-3)18(37-5)16(9-12)36-4/h6-10H,11H2,1-5H3,(H2,32,33,34)(H2,25,26,27,28,29)
|
|||
| InChIKey |
GKDRMWXFWHEQQT-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 901119-35-5
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
16775273, 23769915, 75389485, 96026027, 124360793, 125164706, 125299332, 126665637, 134338877, 135262180, 135693729, 136367938, 137245316, 141853032, 144115995, 152159558, 152344305, 160681714, 162038039, 164043535, 164160256, 170501470, 172087031, 172818901, 174006453, 174531355, 185997057, 188941735, 198973453, 206261469, 210274820, 210280453, 223366127, 223388366, 223684830, 227435155, 242060176, 246229128, 251971029, 252215442
|
|||
| ADReCS Drug ID | BADD_D02499 | |||
| Interaction between the Drug and Microbe | Top | |||
|---|---|---|---|---|
| The Metabolism of Drug Affected by Studied Microbe(s) | ||||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Gut microbiota | ||||
| Studied Microbe: Anaerobic bacterium unspecific | [4], [5] | |||
| Metabolic Reaction | O-demethylation | |||
| Resulting Metabolite | R529 | |||
| Description | Fostamatinib can be metabolized to R529 by unspecific Anaerobic bacterium through O-demethylation. | |||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | 2018 FDA drug approvals.Nat Rev Drug Discov. 2019 Feb;18(2):85-89. | |||
| REF 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7796). | |||
| REF 3 | ClinicalTrials.gov (NCT01197521) Evaluation of Effectiveness of Two Dosing Regimens of Fostamatinib Compared to Placebo in Patients With Rheumatoid Arthritis (RA) Who Are Taking Methotrexate But Not Responding.. U.S. National Institutes of Health. | |||
| REF 4 | Gut microbiome interactions with drug metabolism, efficacy, and toxicity. Transl Res. 2017 Jan;179:204-222. | |||
| REF 5 | Metabolism of fostamatinib, the oral methylene phosphate prodrug of the spleen tyrosine kinase inhibitor R406 in humans: contribution of hepatic and gut bacterial processes to the overall biotransformation. Drug Metab Dispos. 2010 Jul;38(7):1166-76. | |||
| REF 6 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 2230). | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

