Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0U5UC
|
|||
| Former ID |
DIB006339
|
|||
| Drug Name |
Belumosudil
|
|||
| Synonyms |
KD025
Click to Show/Hide
|
|||
| Indication | Graft-versus-host disease [ICD-11: 4B24; ICD-9: 279.5] | Approved | [1] | |
| Psoriasis vulgaris [ICD-11: EA90; ICD-9: 696] | Phase 2 | [2] | ||
| Rheumatoid arthritis [ICD-11: FA20] | Phase 2 | [3] | ||
| Psoriatic arthritis [ICD-11: FA21; ICD-9: 696] | Phase 1 | [4] | ||
| Scleroderma [ICD-11: 4A42; ICD-9: 701.0710.1] | Phase 1 | [4] | ||
| Systemic lupus erythematosus [ICD-11: 4A40.0; ICD-9: 710] | Phase 1 | [4] | ||
| Company |
Kadmon
|
|||
| Structure |
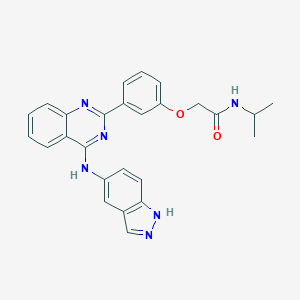 |
Download2D MOL |
||
| Formula |
C26H24N6O2
|
|||
| Canonical SMILES |
CC(C)NC(=O)COC1=CC=CC(=C1)C2=NC3=CC=CC=C3C(=N2)NC4=CC5=C(C=C4)NN=C5
|
|||
| InChI |
1S/C26H24N6O2/c1-16(2)28-24(33)15-34-20-7-5-6-17(13-20)25-30-23-9-4-3-8-21(23)26(31-25)29-19-10-11-22-18(12-19)14-27-32-22/h3-14,16H,15H2,1-2H3,(H,27,32)(H,28,33)(H,29,30,31)
|
|||
| InChIKey |
GKHIVNAUVKXIIY-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 911417-87-3
|
|||
| PubChem Compound ID | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | FDA Approved Drug Products from FDA Official Website. 2021. Application Number: 214783. | |||
| REF 2 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 3 | ClinicalTrials.gov (NCT02317627) Study of of KD025 in Subjects With Psoriasis Vulgaris Who Failed First-Line Therapy. U.S. National Institutes of Health. | |||
| REF 4 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 5 | Selective oral ROCK2 inhibitor down-regulates IL-21 and IL-17 secretion in human T cells via STAT3-dependent mechanism. Proc Natl Acad Sci U S A. 2014 Nov 25;111(47):16814-9. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

