Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0Q5XX
|
|||
| Former ID |
DNCL001818
|
|||
| Drug Name |
Eicosapentaenoic acid/docosa-hexaenoic acid
|
|||
| Synonyms |
Docosahexaenoic acid; Doconexent; Cervonic acid; 6217-54-5; all-cis-DHA; Doconexentum; Doconexento; Doxonexent; (4Z,7Z,10Z,13Z,16Z,19Z)-docosa-4,7,10,13,16,19-hexaenoic acid; AquaGrow Advantage; all-Z-Docosahexaenoic acid; Martek DHA HM; Ropufa 60; cis-4,7,10,13,16,19-Docosahexaenoic acid; Docosahexaenoate; UNII-ZAD9OKH9JC; (4Z,7Z,10Z,13Z,16Z,19Z)-Docosahexaenoic acid; Docosahexaenoic acid (all-Z); CCRIS 7670; all-cis-4,7,10,13,16,19-Docosahexaenoic acid; ZAD9OKH9JC; all-cis-docosa-4,7,10,13,16,19-hexaenoic acid; CHEMBL367149; Espanova (TN); DOCOSAHEXAENOIC ACID
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Hypertriglyceridemia [ICD-11: 5C80.1; ICD-10: E78.1, E78.3; ICD-9: 272.1, 427] | Approved | [1] | |
| Alzheimer disease [ICD-11: 8A20; ICD-10: G30, G30.9; ICD-9: 331] | Phase 3 | [2] | ||
| Company |
Omthera Pharmaceutials
|
|||
| Structure |
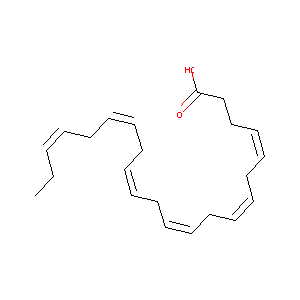 |
Download2D MOL |
||
| Formula |
C22H32O2
|
|||
| Canonical SMILES |
CCC=CCC=CCC=CCC=CCC=CCC=CCCC(=O)O
|
|||
| InChI |
1S/C22H32O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20-21-22(23)24/h3-4,6-7,9-10,12-13,15-16,18-19H,2,5,8,11,14,17,20-21H2,1H3,(H,23,24)/b4-3-,7-6-,10-9-,13-12-,16-15-,19-18-
|
|||
| InChIKey |
MBMBGCFOFBJSGT-KUBAVDMBSA-N
|
|||
| CAS Number |
CAS 6217-54-5
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
8664, 839059, 841847, 7850049, 8144392, 10299728, 14924254, 24893696, 26754926, 26754927, 26754928, 36888254, 46391883, 47515089, 47515090, 47885165, 48184752, 50110834, 50110835, 57404690, 75281905, 81063745, 85787686, 87568664, 91695933, 92165132, 92298371, 92309714, 92719165, 99300628, 99302146, 103445456, 104046523, 104086818, 104179148, 104634620, 118048648, 126524415, 126621028, 126655302, 134350834, 135028589, 135651477, 137003172, 142339426, 144205545, 152101578, 162180738, 162227670, 163659264
|
|||
| ChEBI ID |
CHEBI:28125
|
|||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | FDA approves EPANOVA for the treatment of adults with severe hypertriglyceridemia | |||
| REF 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 1051). | |||
| REF 3 | Cox-2 inhibitory effects of naturally occurring and modified fatty acids. J Nat Prod. 2001 Jun;64(6):745-9. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

