Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0O9GJ
|
|||
| Former ID |
DIB018810
|
|||
| Drug Name |
alphabeta-methyleneADP
|
|||
| Synonyms |
Phosphomethylphosphonic acid adenosyl ester; Adenosine 5'-methylenediphosphate; phosphomethylphosphonic acid adenosyl ester; 3768-14-7; alpha,beta-methylene ADP; MethADP; AMP-CP; UNII-0T2A5439OE; Adenosine, 5'-(trihydrogen methylenebis(phosphonate)); EINECS 223-194-0; NSC 614641; ALPHA,BETA-METHYLENEADENOSINE 5'-DIPHOSPHATE; Adenosine 5'-(hydrogen (phosphonomethyl)phosphonate); CHEBI:40730; 0T2A5439OE; ALPHA,BETA-METHYLENEADENOSINE-5'-DIPHOSPHATE; AMPCP; 5'-O-[hydroxy(phosphonomethyl)phosphoryl]adenosine; Adenosine 5'-(alpha,beta-methylene)diphosphoric acid; alpha,beta-methyleneadenosine 5'-diphosphate (alphabetameADP); [alphabetaCH2]ADP; Phosphomethylphosphonic Acid Adenosyl Ester
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Discovery agent [ICD-11: N.A.] | Investigative | [1], [2] | |
| Structure |
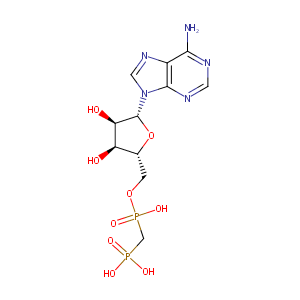 |
Download2D MOL |
||
| Formula |
C11H17N5O9P2
|
|||
| Canonical SMILES |
C1=NC(=C2C(=N1)N(C=N2)C3C(C(C(O3)COP(=O)(CP(=O)(O)O)O)O)O)N
|
|||
| InChI |
1S/C11H17N5O9P2/c12-9-6-10(14-2-13-9)16(3-15-6)11-8(18)7(17)5(25-11)1-24-27(22,23)4-26(19,20)21/h2-3,5,7-8,11,17-18H,1,4H2,(H,22,23)(H2,12,13,14)(H2,19,20,21)/t5-,7-,8-,11-/m1/s1
|
|||
| InChIKey |
OLCWZBFDIYXLAA-IOSLPCCCSA-N
|
|||
| CAS Number |
CAS 3768-14-7
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
822018, 826841, 837272, 7885577, 7885916, 10225253, 11532930, 15056410, 24896872, 26744166, 26756681, 44423060, 46518245, 50064745, 51091073, 57335171, 76270692, 85149275, 85197808, 85197812, 85756529, 92098536, 103696599, 104406789, 123059313, 128824729, 131349752, 135049476, 136349766, 136349770, 137263091, 139404062, 160644369, 160780821, 162227601, 163124585, 163831629, 178101785, 204356569, 230255671, 241076342, 252319262, 252344743
|
|||
| ChEBI ID |
CHEBI:40730
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Ecto-5'-nucleotidase (CD73) | Target Info | Inhibitor | [3] |
| Human Deoxyribonucleic acid (hDNA) | Target Info | Inhibitor | [2] | |
| BioCyc | Purine nucleotides degradation | |||
| Urate biosynthesis/inosine 5'-phosphate degradation | ||||
| Adenosine nucleotides degradation | ||||
| KEGG Pathway | Purine metabolism | |||
| Pyrimidine metabolism | ||||
| Nicotinate and nicotinamide metabolism | ||||
| Metabolic pathways | ||||
| Panther Pathway | Purine metabolism | |||
| Pyrimidine Metabolism | ||||
| Pathway Interaction Database | HIF-1-alpha transcription factor network | |||
| Reactome | Purine catabolism | |||
| WikiPathways | Differentiation Pathway | |||
| miR-targeted genes in muscle cell - TarBase | ||||
| miR-targeted genes in lymphocytes - TarBase | ||||
| miR-targeted genes in epithelium - TarBase | ||||
| Metabolism of nucleotides | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5092). | |||
| REF 2 | How many drug targets are there Nat Rev Drug Discov. 2006 Dec;5(12):993-6. | |||
| REF 3 | 5'-Nucleotidase from smooth muscle of small intestine and from brain. Inhibition of nucleotides. Biochemistry. 1975 Jun 3;14(11):2362-6. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

