Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0O6SI
|
|||
| Former ID |
DNC003041
|
|||
| Drug Name |
1,4-Butanediol
|
|||
| Synonyms |
1,4-BUTANEDIOL; Butane-1,4-diol; 110-63-4; 1,4-Butylene glycol; Tetramethylene glycol; 1,4-Dihydroxybutane; 1,4-Tetramethylene glycol; Tetramethylene 1,4-diol; Sucol B; 1,4-BD; DIOL 14B; Agrisynth B1D; UNII-7XOO2LE6G3; HO(CH2)4OH; NSC 406696; CCRIS 5984; 1,4-Dihdyroxybutane; HSDB 1112; EINECS 203-786-5; HOCH2CH2CH2CH2OH; BRN 1633445; 7XOO2LE6G3; AI3-07553; CHEBI:41189; WERYXYBDKMZEQL-UHFFFAOYSA-N; MFCD00002968; DSSTox_CID_4666; DSSTox_RID_77492; DSSTox_GSID_24666; BDO; CAS-110-63-4; BU1; 1,4-Butanediol, homopolymer; 4-hydroxybutanol
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Discovery agent [ICD-11: N.A.] | Investigative | [1] | |
| Structure |
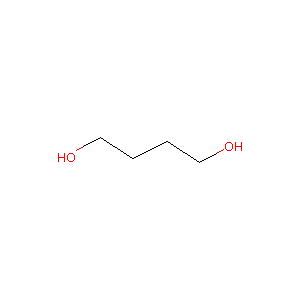 |
Download2D MOL |
||
| Formula |
C4H10O2
|
|||
| Canonical SMILES |
C(CCO)CO
|
|||
| InChI |
1S/C4H10O2/c5-3-1-2-4-6/h5-6H,1-4H2
|
|||
| InChIKey |
WERYXYBDKMZEQL-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 110-63-4
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
476590, 584198, 587704, 826407, 826408, 833916, 3132270, 5759543, 7886370, 8155604, 10317707, 10317714, 10503864, 12077028, 15194210, 17389457, 17435958, 24854421, 24861305, 24861306, 24861307, 24861310, 24861311, 24866368, 24872855, 24874546, 24886097, 24886229, 24886474, 24887581, 24889295, 24889502, 24889905, 24889911, 26703409, 26703656, 26750555, 32679244, 37626313, 38236237, 38646019, 39838688, 46506248, 48417245, 48422135, 48424398, 49703992, 49816801, 49854712, 56465060
|
|||
| ChEBI ID |
CHEBI:41189
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Group IIA phospholipase A2 (GIIA sPLA2) | Target Info | Inhibitor | [1] |
| BioCyc | Phospholipases | |||
| KEGG Pathway | Glycerophospholipid metabolism | |||
| Ether lipid metabolism | ||||
| Arachidonic acid metabolism | ||||
| Linoleic acid metabolism | ||||
| alpha-Linolenic acid metabolism | ||||
| Metabolic pathways | ||||
| Ras signaling pathway | ||||
| Vascular smooth muscle contraction | ||||
| Pancreatic secretion | ||||
| Fat digestion and absorption | ||||
| Pathway Interaction Database | Glypican 1 network | |||
| Reactome | Acyl chain remodelling of PC | |||
| Acyl chain remodelling of PE | ||||
| Acyl chain remodelling of PI | ||||
| WikiPathways | Cardiac Hypertrophic Response | |||
| Glycerophospholipid biosynthesis | ||||
| Glycerophospholipid Biosynthetic Pathway | ||||
| Spinal Cord Injury | ||||
| Eicosanoid Synthesis | ||||
| MicroRNAs in cardiomyocyte hypertrophy | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Drosophila metabolize 1,4-butanediol into gamma-hydroxybutyric acid in vivo. Eur J Pharmacol. 2003 Jul 25;473(2-3):149-52. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

