Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0NU0U
|
|||
| Former ID |
DNCL002653
|
|||
| Drug Name |
AZD4547
|
|||
| Synonyms |
AZD4547; 1035270-39-3; AZD-4547; AZD 4547; UNII-2167OG1EKJ; 2167OG1EKJ; CHEBI:63453; KB-74810; N-(5-(3,5-Dimethoxyphenethyl)-1H-pyrazol-3-yl)-4-((3R,5S)-rel-3,5-dimethylpiperazin-1-yl)benzamide; N-{5-[2-(3,5-dimethoxyphenyl)ethyl]-1H-pyrazol-3-yl}-4-(cis-3,5-dimethylpiperazin-1-yl)benzamide; rel-N-[5-[2-(3,5-Dimethoxyphenyl)ethyl]-1H-pyrazol-3-yl]-4-[(3R,5S)-3,5-dimethyl-1-piperazinyl]benzamide; N-[5-[2-(3,5-dimethoxyphenyl)ethyl]-1H-pyrazol-3-yl]-4-[(3R,5S)-3,5-dimethylpiperazin-1-yl]benzamide
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Solid tumour/cancer [ICD-11: 2A00-2F9Z; ICD-10: C00-D48; ICD-9: 140-199, 210-229] | Phase 2/3 | [1], [2] | |
| Company |
AstraZeneca
|
|||
| Structure |
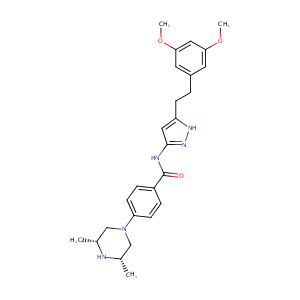 |
Download2D MOL |
||
| Formula |
C26H33N5O3
|
|||
| Canonical SMILES |
CC1CN(CC(N1)C)C2=CC=C(C=C2)C(=O)NC3=NNC(=C3)CCC4=CC(=CC(=C4)OC)OC
|
|||
| InChI |
1S/C26H33N5O3/c1-17-15-31(16-18(2)27-17)22-9-6-20(7-10-22)26(32)28-25-13-21(29-30-25)8-5-19-11-23(33-3)14-24(12-19)34-4/h6-7,9-14,17-18,27H,5,8,15-16H2,1-4H3,(H2,28,29,30,32)/t17-,18+
|
|||
| InChIKey |
VRQMAABPASPXMW-HDICACEKSA-N
|
|||
| CAS Number |
CAS 1035270-39-3
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
117699613, 135610501, 136340324, 136350053, 136920281, 136970544, 140819315, 144115667, 152159627, 163643100, 164041821, 172918164, 174006594, 174525910, 185997025, 190361859, 193883042, 198940588, 223366040, 223367238, 223404365, 223436471, 223471434, 223702523, 224188020, 226445213, 240205758, 241383730, 242587692, 248906639, 249818642, 249869575, 252110149, 252160568, 252442834, 252451807, 252470587
|
|||
| ChEBI ID |
CHEBI:63453
|
|||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7707). | |||
| REF 2 | ClinicalTrials.gov (NCT02154490) Lung-MAP: S1400 Biomarker-Targeted Second-Line Therapy in Treating Patients With Recurrent Stage IIIB-IV Squamous Cell Lung Cancer. U.S. National Institutes of Health. | |||
| REF 3 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 4 | AZD4547: an orally bioavailable, potent, and selective inhibitor of the fibroblast growth factor receptor tyrosine kinase family. Cancer Res. 2012 Apr 15;72(8):2045-56. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

