Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0LQ3L
|
|||
| Drug Name |
GSK2982772
|
|||
| Synonyms |
LYPAFUINURXJSG-AWEZNQCLSA-N; 1622848-92-3; UNII-T5W3M0VO9B; T5W3M0VO9B; GSK-2982772; (S)-5-benzyl-N-(5-methyl-4-oxo-2,3,4,5-tetrahydrobenzo[b][1,4]oxazepin-3-yl)-1H-1,2,4-triazole-3-carboxamide; GTPL9554; SCHEMBL15956219; MolPort-044-830-634; s8484; AKOS030528033; compound 5 [PMID: 28151659]; ACN-041458; CS-6899; GSK 2982772; AS-35128; AC-29894; HY-101760; J3.650.802G; 5-benzyl-N-[(3S)-5-methyl-4-oxo-2,3-dihydro-1,5-benzoxazepin-3-yl]-1H-1,2,4-triazole-3-carboxamide; 3-Benzyl-N-[(3s)-5-Methyl-4-Oxo-2,3,4,5-Tetrahydr
Click to Show/Hide
|
|||
| Indication | Plaque psoriasis [ICD-11: EA90.0; ICD-10: L40.0] | Phase 2 | [1] | |
| Psoriasis vulgaris [ICD-11: EA90; ICD-9: 696] | Phase 1 | [2] | ||
| Rheumatoid arthritis [ICD-11: FA20] | Phase 1 | [2] | ||
| Ulcerative colitis [ICD-11: DD71; ICD-9: 556] | Phase 1 | [2] | ||
| Company |
GlaxoSmithKline Research Triangle Park, NC
|
|||
| Structure |
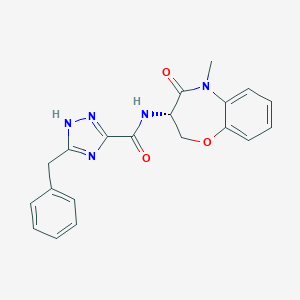 |
Download2D MOL |
||
| Formula |
C20H19N5O3
|
|||
| Canonical SMILES |
CN1C2=CC=CC=C2OCC(C1=O)NC(=O)C3=NNC(=N3)CC4=CC=CC=C4
|
|||
| InChI |
1S/C20H19N5O3/c1-25-15-9-5-6-10-16(15)28-12-14(20(25)27)21-19(26)18-22-17(23-24-18)11-13-7-3-2-4-8-13/h2-10,14H,11-12H2,1H3,(H,21,26)(H,22,23,24)/t14-/m0/s1
|
|||
| InChIKey |
LYPAFUINURXJSG-AWEZNQCLSA-N
|
|||
| CAS Number |
CAS 1622848-92-3
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Receptor-interacting protein 1 (RIPK1) | Target Info | Inhibitor | [1], [2] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 2 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

