Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0K2ER
|
|||
| Former ID |
DNC000953
|
|||
| Drug Name |
MK-886
|
|||
| Synonyms |
MK-886; 118414-82-7; MK 886; MK886; 3-(3-(tert-butylthio)-1-(4-chlorobenzyl)-5-isopropyl-1H-indol-2-yl)-2,2-dimethylpropanoic acid; UNII-080626SQ8C; QAOAOVKBIIKRNL-UHFFFAOYSA-N; L-663,536; CHEMBL29097; CHEBI:75390; L 663536; MK-886 (L-663,536); 080626SQ8C; 3-[3-tert-butylsulfanyl-1-[(4-chlorophenyl)methyl]-5-propan-2-ylindol-2-yl]-2,2-dimethylpropanoic acid; 1H-Indole-2-propanoic acid, 1-[(4-chlorophenyl)methyl]-3-[(1,1-dimethylethyl)thio]-Alpha,Alpha-dimethyl-5-(1-methylethyl)- [CAS]; L-663536; SMR000466278
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Asthma [ICD-11: CA23; ICD-10: J45, J45.8; ICD-9: 493] | Discontinued in Phase 2 | [1], [2] | |
| Structure |
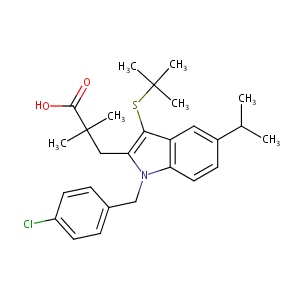 |
Download2D MOL |
||
| Formula |
C27H34ClNO2S
|
|||
| Canonical SMILES |
CC(C)C1=CC2=C(C=C1)N(C(=C2SC(C)(C)C)CC(C)(C)C(=O)O)CC3=CC=C(C=C3)Cl
|
|||
| InChI |
1S/C27H34ClNO2S/c1-17(2)19-10-13-22-21(14-19)24(32-26(3,4)5)23(15-27(6,7)25(30)31)29(22)16-18-8-11-20(28)12-9-18/h8-14,17H,15-16H2,1-7H3,(H,30,31)
|
|||
| InChIKey |
QAOAOVKBIIKRNL-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 118414-82-7
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
4980721, 9827559, 11114025, 14858675, 26719785, 37846566, 46386984, 47500599, 47870808, 48431170, 49681739, 53790634, 56313866, 78784119, 91746362, 92308508, 103202029, 103920123, 112031129, 124659039, 124887007, 125334114, 128995122, 135061508, 135650618, 135698297, 137035287, 138032501, 162012057, 162022262, 164175058, 172080426, 172086198, 172111546, 179149662, 215777559, 223536000, 224095260, 226817470, 241182320, 242060382, 250136881
|
|||
| ChEBI ID |
CHEBI:75390
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Arachidonate 5-lipoxygenase (5-LOX) | Target Info | Modulator | [3] |
| BioCyc | Aspirin-triggered lipoxin biosynthesis | |||
| Resolvin D biosynthesis | ||||
| Leukotriene biosynthesis | ||||
| Lipoxin biosynthesis | ||||
| Aspirin triggered resolvin D biosynthesis | ||||
| Aspirin triggered resolvin E biosynthesis | ||||
| KEGG Pathway | Arachidonic acid metabolism | |||
| Metabolic pathways | ||||
| Serotonergic synapse | ||||
| Ovarian steroidogenesis | ||||
| Toxoplasmosis | ||||
| NetPath Pathway | IL4 Signaling Pathway | |||
| Pathwhiz Pathway | Arachidonic Acid Metabolism | |||
| WikiPathways | Vitamin D Receptor Pathway | |||
| Arachidonic acid metabolism | ||||
| Eicosanoid Synthesis | ||||
| Selenium Micronutrient Network | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2655). | |||
| REF 2 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800000073) | |||
| REF 3 | Inhibitory effects of MK-886 on arachidonic acid metabolism in human phagocytes. Br J Pharmacol. 1990 May;100(1):15-20. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

