Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0ED7U
|
|||
| Former ID |
DAP000164
|
|||
| Drug Name |
Tiagabine
|
|||
| Synonyms |
tiagabine; 115103-54-3; Gabitril; Tiagabinum; Tiagabina; (R)-Tiagabine; Tiagabinum [INN-Latin]; Tiagabina [INN-Spanish]; Tiagabine [INN:BAN]; Tiagabine [INN]; Abbott-70569; UNII-Z80I64HMNP; Tiagabine (INN); ABBOTT-70569-1; Gabitril (TN); CHEMBL1027; Z80I64HMNP; ABT-569; CHEBI:9586; NO-328; (-)-(R)-1-(4,4-Bis(3-methyl-2-thienyl)-3-butenyl)nipecotic acid; A-70569-1; (R)-1-(4,4-Bis(3-methylthiophen-2-yl)but-3-en-1-yl)piperidine-3-carboxylic acid; Gabatril; NO 329; Gabitril; NO-329; Tiagabina [INN-Spanish];Tiagabine (INN); N-(4,4-di(3-methylthien-2-yl)but-3-enyl)nipecotic acid; (3R)-1-[4,4-bis(3-methyl-2-thienyl)but-3-en-1-yl]piperidine-3-carboxylic acid; (3R)-1-[4,4-bis(3-methylthiophen-2-yl)but-3-enyl]piperidine-3-carboxylic acid; [3H]tiagabine
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Epilepsy [ICD-11: 8A60-8A68] | Approved | [1], [2] | |
| Anxiety disorder [ICD-11: 6B00-6B0Z] | Phase 2 | [1], [2] | ||
| Therapeutic Class |
Anticonvulsants
|
|||
| Company |
Cephalon
|
|||
| Structure |
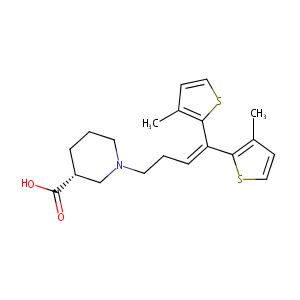 |
Download2D MOL |
||
| Formula |
C20H25NO2S2
|
|||
| Canonical SMILES |
CC1=C(SC=C1)C(=CCCN2CCCC(C2)C(=O)O)C3=C(C=CS3)C
|
|||
| InChI |
1S/C20H25NO2S2/c1-14-7-11-24-18(14)17(19-15(2)8-12-25-19)6-4-10-21-9-3-5-16(13-21)20(22)23/h6-8,11-12,16H,3-5,9-10,13H2,1-2H3,(H,22,23)/t16-/m1/s1
|
|||
| InChIKey |
PBJUNZJWGZTSKL-MRXNPFEDSA-N
|
|||
| CAS Number |
CAS 115103-54-3
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
9706, 7980788, 8186978, 14804309, 14926800, 26758048, 43118012, 46505560, 48416622, 50015636, 50510996, 53787330, 92723327, 93166176, 96025272, 103276854, 103946490, 104321287, 117542112, 126592912, 126617580, 128891680, 134337484, 135016989, 137002486, 142970946, 160847730, 160964245, 163418753, 163850077, 164814870, 172858819, 175268881, 178101399, 178101520, 179116918, 184545901, 210279817, 210282140, 223441308, 223554966, 223655564, 224165587, 226421082, 251916677, 251917916, 251970975, 252357544
|
|||
| ChEBI ID |
CHEBI:9586
|
|||
| ADReCS Drug ID | BADD_D02210 ; BADD_D02211 | |||
| SuperDrug ATC ID |
N03AG06
|
|||
| SuperDrug CAS ID |
cas=115103543
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | GABA transporter GAT-1 (SLC6A1) | Target Info | Inhibitor | [3] |
| Gamma-aminobutyric acid uptake (GABAU) | Target Info | Inhibitor | [4] | |
| KEGG Pathway | GABAergic synapse | |||
| Reactome | Na+/Cl- dependent neurotransmitter transporters | |||
| WikiPathways | Monoamine Transport | |||
| NRF2 pathway | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 4818). | |||
| REF 2 | Use of second-generation antiepileptic drugs in the pediatric population. Paediatr Drugs. 2008;10(4):217-54. | |||
| REF 3 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 929). | |||
| REF 4 | Glutamate- and GABA-based CNS therapeutics. Curr Opin Pharmacol. 2006 Feb;6(1):7-17. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

