Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0E0NM
|
|||
| Former ID |
DNCL002117
|
|||
| Drug Name |
DCC-2036
|
|||
| Synonyms |
REBASTINIB; DCC-2036; 1020172-07-9; DCC-2036 (Rebastinib); DCC 2036; CHEBI:62166; UNII-75017Q6I97; Rebastinib(DCC-2036); 4-(4-(3-(3-(tert-butyl)-1-(quinolin-6-yl)-1H-pyrazol-5-yl)ureido)-3-fluorophenoxy)-N-methylpicolinamide; DCC2036; DP-1919; 4-[4-({[3-Tert-Butyl-1-(Quinolin-6-Yl)-1h-Pyrazol-5-Yl]carbamoyl}amino)-3-Fluorophenoxy]-N-Methylpyridine-2-Carboxamide; 75017Q6I97; 4-[4-[(5-Tert-butyl-2-quinolin-6-ylpyrazol-3-yl)carbamoylamino]-3-fluorophenoxy]-N-methylpyridine-2-carboxamide,4-methylbenzenesulfonic acid;4-[4-[(
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Chronic myeloid leukaemia [ICD-11: 2A20; ICD-10: C91-C95; ICD-9: 208.9] | Phase 1/2 | [1] | |
| Acute lymphoblastic leukaemia [ICD-11: 2A85; ICD-10: C85.1, C88.7] | Phase 1 | [1] | ||
| Breast cancer [ICD-11: 2C60-2C65] | Phase 1 | [2] | ||
| Company |
Deciphera Pharmaceuticals
|
|||
| Structure |
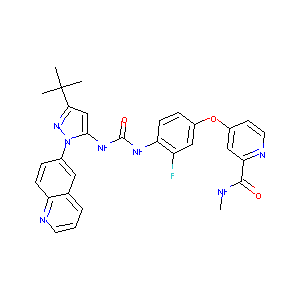 |
Download2D MOL |
||
| Formula |
C30H28FN7O3
|
|||
| Canonical SMILES |
CC(C)(C)C1=NN(C(=C1)NC(=O)NC2=C(C=C(C=C2)OC3=CC(=NC=C3)C(=O)NC)F)C4=CC5=C(C=C4)N=CC=C5
|
|||
| InChI |
1S/C30H28FN7O3/c1-30(2,3)26-17-27(38(37-26)19-7-9-23-18(14-19)6-5-12-33-23)36-29(40)35-24-10-8-20(15-22(24)31)41-21-11-13-34-25(16-21)28(39)32-4/h5-17H,1-4H3,(H,32,39)(H2,35,36,40)
|
|||
| InChIKey |
WVXNSAVVKYZVOE-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 1020172-07-9
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
56403339, 57548987, 123055185, 123055186, 123058785, 124958086, 136349530, 136367661, 136920318, 137276016, 144115890, 160644569, 162011946, 162037711, 162108915, 162202690, 163620736, 163686053, 164045127, 170483879, 170483880, 172232428, 185990477, 189622837, 198983986, 223389164, 223705271, 228188520, 245535818, 249814481, 252110192, 252110222, 252160750, 252216186, 252451795
|
|||
| ChEBI ID |
CHEBI:62166
|
|||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT00827138) Study Safety and Preliminary Efficacy of DCC-2036 in Patients With Leukemias (Ph+ CML With T315I Mutation). U.S. National Institutes of Health. | |||
| REF 2 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 3 | Company report (Deciphera Pharmaceuticals: Tumor-Targeted Programs and Indications) | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

