Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0D9IQ
|
|||
| Former ID |
DIB012426
|
|||
| Drug Name |
BGB-290
|
|||
| Synonyms |
Pamiparib
Click to Show/Hide
|
|||
| Indication | Ovarian cancer [ICD-11: 2C73; ICD-10: C56; ICD-9: 183] | Phase 2 | [1] | |
| Solid tumour/cancer [ICD-11: 2A00-2F9Z; ICD-10: C00-D48; ICD-9: 140-199, 210-229] | Phase 1/2 | [2] | ||
| Recurrent glioblastoma [ICD-11: 2A00.00; ICD-10: C71] | Phase 1 | [2], [3] | ||
| Structure |
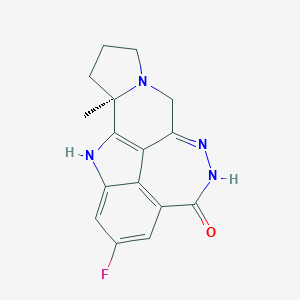 |
Download2D MOL |
||
| Formula |
C16H15FN4O
|
|||
| Canonical SMILES |
CC12CCCN1CC3=NNC(=O)C4=C5C3=C2NC5=CC(=C4)F
|
|||
| InChI |
1S/C16H15FN4O/c1-16-3-2-4-21(16)7-11-13-12-9(15(22)20-19-11)5-8(17)6-10(12)18-14(13)16/h5-6,18H,2-4,7H2,1H3,(H,20,22)/t16-/m1/s1
|
|||
| InChIKey |
DENYZIUJOTUUNY-MRXNPFEDSA-N
|
|||
| CAS Number |
CAS 1446261-44-4
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Poly [ADP-ribose] polymerase (PARP) | Target Info | Inhibitor | [2], [3] |
| Tankyrase (TNKS) | Target Info | Modulator | [4] | |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT03933761) Pamiparib in Fusion Positive, Reversion Negative High Grade Serous Ovarian Cancer or Carcinosarcoma With BRCA1/2 Gene Mutations If Progression on Substrate Poly ADP Ribose Polymerase Inhibitbor (PARPI) or Chemotherapy (PRECISE). U.S. National Institutes of Health. | |||
| REF 2 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 3 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 4 | National Cancer Institute Drug Dictionary (drug id 769217). | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

