Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0B8SV
|
|||
| Former ID |
DAP001291
|
|||
| Drug Name |
NADH
|
|||
| Synonyms |
DPNH; Dihydrodiphosphopyridine nucleotide; Dihydronicotinamide adenine dinucleotide; Diphosphopyridine nucleotide reduced; NADH dianion; Nicotinamide adenine dinucleotide reduced; Reduced Nicotinamide Adenine Dinucleotide; NADH2; Beta-DPNH; Beta-NADH; Coenzyme I, reduced; Cozymase I, reduced; Diphosphopyridine nucleotide,reduced form; NAD-reduced; Nicotinamide adenine dinucleotide, reduced form; Reduced nicotinamide-adenine dinucleotide; Beta-Nicotinamide adenine dinucleotide, reduced dipotassium salt; NADH+H+; Nicotinaminde-Adenine-Dinucleotide; Adenosine pyrophosphate, 5'->5'-ester with 1,4-dihydro-1-beta-D-ribofuranosylnicotinamide (7CI); Adenosine 5'-(trihydrogen pyrophosphate), 5'->5'-ester with 1,4-dihydro-1-beta-D-ribofuranosylnicotinamide (8CI); Adenosine 5'-(trihydrogen diphosphate), P'->5'-ester with 1,4-dihydro-1-beta-D-ribofuranosyl-3-pyridinecarboxamide (9CI); Adenosine 5'-{3-[1-(3-carbamoyl-1,4-dihydropyridin-1-yl)-1,4-anhydro-D-ribitol-5-yl] dihydrogen diphosphate}; Adenosine 5'-{3-[1-(3-carbamoyl-1,4-dihydropyridin-1-yl)-1,4-anhydro-D-ribitol-5-yl] diphosphate}; [5-(6-Aminopurin-9-yl)-3,4-dihydroxyoxolan-2-yl]methyl [[5-(3-carbamoyl-4H-pyridin-1-yl)-3,4-dihydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl] hydrogen phosphate; [[(2R,3S,4R,5R)-5-(6-aminopurin-9-yl)-3,4-dihydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl] [(2R,3S,4R,5R)-5-(3-carbamoyl-4H-pyridin-1-yl)-3,4-dihydroxyoxolan-2-yl]methyl hydrogen phosphate; [(2R,3S,4R,5R)-5-(6-Aminopurin-9-yl)-3,4-dihydroxy-oxolan-2-yl]methoxy-[[(2R,3S,4R,5R)-5-(3-carbamoyl-4H-pyridin-1-yl)-3,4-dihydroxy-oxolan-2-yl]methoxy-hydroxy-phosphoryl]oxy-phosphinic acid
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Parkinson disease [ICD-11: 8A00.0; ICD-9: 332] | Approved | [1], [2] | |
| Therapeutic Class |
Dietary supplement
|
|||
| Structure |
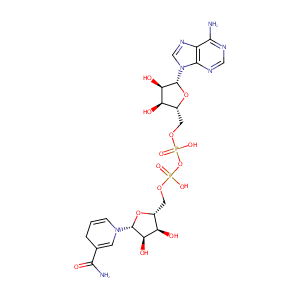 |
Download2D MOL |
||
| Formula |
C21H29N7O14P2
|
|||
| Canonical SMILES |
C1C=CN(C=C1C(=O)N)C2C(C(C(O2)COP(=O)(O)OP(=O)(O)OCC3C(C(C(O3)N4C=NC5=C(N=CN=C54)N)O)O)O)O
|
|||
| InChI |
1S/C21H29N7O14P2/c22-17-12-19(25-7-24-17)28(8-26-12)21-16(32)14(30)11(41-21)6-39-44(36,37)42-43(34,35)38-5-10-13(29)15(31)20(40-10)27-3-1-2-9(4-27)18(23)33/h1,3-4,7-8,10-11,13-16,20-21,29-32H,2,5-6H2,(H2,23,33)(H,34,35)(H,36,37)(H2,22,24,25)/t10-,11-,13-,14-,15-,16-,20-,21-/m1/s1
|
|||
| InChIKey |
BOPGDPNILDQYTO-NNYOXOHSSA-N
|
|||
| CAS Number |
CAS 58-68-4
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
3306, 584476, 584809, 585516, 821913, 823134, 825930, 825936, 828466, 828744, 828908, 841526, 8024318, 8145742, 10298218, 10317217, 14839494, 24775903, 26702311, 26704397, 26706856, 26707742, 26707751, 26707759, 26708871, 26711774, 26712883, 36883413, 46504879, 50172602, 57402874, 81059101, 81060441, 85164328, 85164331, 85164902, 85176956, 104619760, 123059890, 123059893, 123059898, 127284280, 127284281, 127284282, 127284283, 127284284, 127284285, 127284286, 127284287, 127284288
|
|||
| ChEBI ID |
CHEBI:16908
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | NADH dehydrogenase (MT-ND3) | Target Info | Binder | [3] |
| KEGG Pathway | Oxidative phosphorylation | |||
| Metabolic pathways | ||||
| Parkinson's disease | ||||
| WikiPathways | Oxidative phosphorylation | |||
| Respiratory electron transport, ATP synthesis by chemiosmotic coupling, and heat production by uncoupling proteins. | ||||
| Electron Transport Chain | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 4487). | |||
| REF 2 | Drug information of NADH, 2008. eduDrugs. | |||
| REF 3 | Mechanism of cadmium-decreased glucuronidation in the rat. Biochem Pharmacol. 1992 Dec 1;44(11):2139-47. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

