Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0A7ZK
|
|||
| Former ID |
DNCL003137
|
|||
| Drug Name |
Lemborexant
|
|||
| Synonyms |
E2006
Click to Show/Hide
|
|||
| Indication | Insomnia [ICD-11: 7A00-7A0Z] | Approved | [1] | |
| Alzheimer disease [ICD-11: 8A20; ICD-10: G30, G30.9; ICD-9: 331] | Phase 2 | [2] | ||
| Structure |
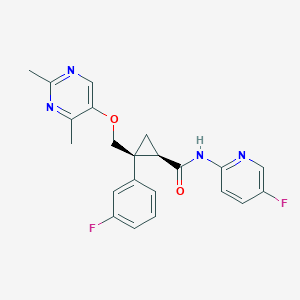 |
Download2D MOL |
||
| Formula |
C22H20F2N4O2
|
|||
| Canonical SMILES |
CC1=NC(=NC=C1OCC2(CC2C(=O)NC3=NC=C(C=C3)F)C4=CC(=CC=C4)F)C
|
|||
| InChI |
1S/C22H20F2N4O2/c1-13-19(11-25-14(2)27-13)30-12-22(15-4-3-5-16(23)8-15)9-18(22)21(29)28-20-7-6-17(24)10-26-20/h3-8,10-11,18H,9,12H2,1-2H3,(H,26,28,29)/t18-,22+/m0/s1
|
|||
| InChIKey |
MUGXRYIUWFITCP-PGRDOPGGSA-N
|
|||
| CAS Number |
CAS 1369764-02-2
|
|||
| PubChem Compound ID | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health Human Services. 2019 | |||
| REF 2 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

