Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0A1AQ
|
|||
| Former ID |
DCL000631
|
|||
| Drug Name |
Rosiglitazone + metformin
|
|||
| Synonyms |
Orantinib; TSU-68; 252916-29-3; SU-6668; SU6668; SU 6668; 210644-62-5; UNII-9RL37ZZ665; Orantinib (TSU-68); NSC 702827; TSU68; CHEMBL274654; 9RL37ZZ665; TSU 68; PDGFR Tyrosine Kinase Inhibitor VI, SU6668; 2,4-Dimethyl-5-[(1,2-dihydro-2-oxo-3H-indol-3-ylidene)methyl]-pyrrole-3-propanoic acid; J-502593; Orantinibum; 3-(2,4-dimethyl-5-{[(3Z)-2-oxo-1H-indol-3-ylidene]methyl}-1H-pyrrol-3-yl)propanoic acid; 3-[2,4-dimethyl-5-[(Z)-(2-oxo-1H-indol-3-ylidene)methyl]-1H-pyrrol-3-yl]propanoic acid; Orantinib [INN]
Click to Show/Hide
|
|||
| Drug Type |
Combination drug (small molecular drug)
|
|||
| Indication | Diabetic complication [ICD-11: 5A2Y; ICD-9: 253.5, 588.1] | Phase 3 | [1] | |
| Advanced solid tumour [ICD-11: 2A00-2F9Z; ICD-9: 140-199] | Phase 1 | [2], [3] | ||
| Company |
GSK
|
|||
| Structure |
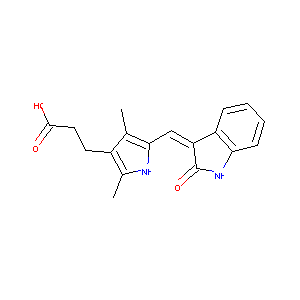 |
Download2D MOL |
||
| Formula |
C18H18N2O3
|
|||
| Canonical SMILES |
CC1=C(NC(=C1CCC(=O)O)C)C=C2C3=CC=CC=C3NC2=O
|
|||
| InChI |
1S/C18H18N2O3/c1-10-12(7-8-17(21)22)11(2)19-16(10)9-14-13-5-3-4-6-15(13)20-18(14)23/h3-6,9,19H,7-8H2,1-2H3,(H,20,23)(H,21,22)/b14-9-
|
|||
| InChIKey |
NHFDRBXTEDBWCZ-ZROIWOOFSA-N
|
|||
| CAS Number |
CAS 210644-62-5
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
527809, 8034459, 11061326, 12015385, 14874233, 39301724, 53787898, 57287542, 57361293, 75555598, 81049299, 89388569, 99437172, 103175695, 113911629, 124757275, 125164079, 134338996, 134339167, 134964221, 135134779, 135182126, 135698723, 136340186, 136348238, 136367533, 136367774, 142063014, 143497361, 152234911, 152258241, 152344216, 160647077, 162011789, 162037617, 162202670, 163685934, 174561004, 177748855, 180386845, 185964372, 198939270, 204362248, 223366147, 223379505, 223705143, 226502638, 241376394, 242060022, 243278051
|
|||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT00499707) Efficacy and Safety Study of Rosiglitazone/Metformin Therapy vs Rosiglitazone and Metformin in Type 2 Diabetes Subjects. U.S. National Institutes of Health. | |||
| REF 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7816). | |||
| REF 3 | A phase I surrogate endpoint study of SU6668 in patients with solid tumors. Invest New Drugs. 2004 Nov;22(4):459-66. | |||
| REF 4 | A comparison of physicochemical property profiles of marketed oral drugs and orally bioavailable anti-cancer protein kinase inhibitors in clinical development. Curr Top Med Chem. 2007;7(14):1408-22. | |||
| REF 5 | Dose-finding study of the multitargeted tyrosine kinase inhibitor SU6668 in patients with advanced malignancies. Clin Cancer Res. 2005 Sep 1;11(17):6240-6. | |||
| REF 6 | Clinical pipeline report, company report or official report of GlaxoSmithKline (2009). | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

