Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D09DHY
|
|||
| Former ID |
DAP001254
|
|||
| Drug Name |
Colchicine
|
|||
| Synonyms |
Colchicin; Colchicina; Colchicinum; Colchineos; Colchisol; Colchysat; Colcin; Colcrys; Colsaloid; Colstat; Condylon; Goutnil; Kolkicin; LOC; Binds to tubulin; Colchicin [German]; Colchicina [Italian]; Colchicine [JAN]; Inhibits microtubular assembly; Spindle poison; C 9754; Colchicine (TN); Colchicine, Colchicum autumnale; MPC-004; N-Acetyl trimethylcolchicinic acid methylether; Colchicine (JP15/USP); Colchicine, (R)-Isomer; Benzo(a)heptalen-9(5H)-one; Colchicine, (+-)-Isomer; N-(5,6,7,9-Tetrahydro-1,2,3,10-tetramethoxy-9-oxobenzo(a)heptalen-7-yl)acetamide; N-[(7S)-1,2,3,10-tetramethoxy-9-oxo-5,6,7,9-tetrahydrobenzo[a]heptalen-7-yl]acetamide; N-[(7S)-1,2,3,10-tetramethoxy-9-oxo-6,7-dihydro-5H-benzo[a]heptalen-7-yl]acetamide; N-[(7S)-5,6,7,9-tetrahydro-1,2,3,10-tetramethoxy-9-oxobenzo[a]heptalen-7-yl]acetamide; N-(5,6,7,9-Tetrahydro-1,2,3,10-tetramethoxy-9-oxobenzo[.alpha.]heptalen-7-yl)-acetamide; N-((7S)-5,6,7,9-tetrahydro-1,2,3,10-tetramethoxy-9-oxobenzo(a)heptalen-7-yl)-acetamide; (S)-N-(5,6,7,9-Tetrahydro-1,2,3,10-tetramethoxy-9-oxobenzo[a]heptalen-7-yl)acetamide; 7-alpha-H-Colchicine; 7.alpha.H-Colchicine; 7alphaH-Colchicine
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Acute gouty arthritis [ICD-11: FA25.0; ICD-10: M10.0; ICD-9: 274] | Approved | [1], [2], [3] | |
| Therapeutic Class |
Gout Suppressants
|
|||
| Structure |
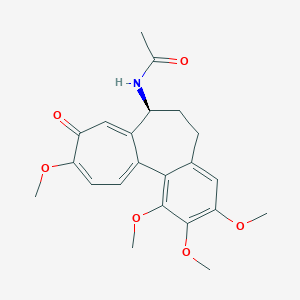 |
Download2D MOL |
||
| Formula |
C22H25NO6
|
|||
| Canonical SMILES |
CC(=O)NC1CCC2=CC(=C(C(=C2C3=CC=C(C(=O)C=C13)OC)OC)OC)OC
|
|||
| InChI |
1S/C22H25NO6/c1-12(24)23-16-8-6-13-10-19(27-3)21(28-4)22(29-5)20(13)14-7-9-18(26-2)17(25)11-15(14)16/h7,9-11,16H,6,8H2,1-5H3,(H,23,24)/t16-/m0/s1
|
|||
| InChIKey |
IAKHMKGGTNLKSZ-INIZCTEOSA-N
|
|||
| CAS Number |
CAS 64-86-8
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| ChEBI ID |
CHEBI:27882
|
|||
| ADReCS Drug ID | BADD_D00518 | |||
| SuperDrug ATC ID |
M04AC01
|
|||
| SuperDrug CAS ID |
cas=000064868
|
|||
| Interaction between the Drug and Microbe | Top | |||
|---|---|---|---|---|
| The Metabolism of Drug Affected by Studied Microbe(s) | ||||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Bacteroidales | ||||
|
Studied Microbe: Bacteroides dorei DSM 17855
Show/Hide Hierarchy
|
[4] | |||
| Hierarchy | ||||
| Experimental Method | High-throughput screening | |||
| Description | Colchicine can be metabolized by Bacteroides dorei DSM 17855 (log2FC = -0.647; p = 0.004). | |||
|
Studied Microbe: Bacteroides fragilis ATCC43859
Show/Hide Hierarchy
|
[4] | |||
| Hierarchy | ||||
| Experimental Method | High-throughput screening | |||
| Description | Colchicine can be metabolized by Bacteroides fragilis ATCC43859 (log2FC = -0.574; p = 0.026). | |||
|
Studied Microbe: Bacteroides fragilis HMW 610
Show/Hide Hierarchy
|
[4] | |||
| Hierarchy | ||||
| Experimental Method | High-throughput screening | |||
| Description | Colchicine can be metabolized by Bacteroides fragilis HMW 610 (log2FC = -0.502; p = 0.01). | |||
|
Studied Microbe: Bacteroides fragilis NCTC 9343
Show/Hide Hierarchy
|
[4] | |||
| Hierarchy | ||||
| Experimental Method | High-throughput screening | |||
| Description | Colchicine can be metabolized by Bacteroides fragilis NCTC 9343 (log2FC = -0.53; p = 0.034). | |||
|
Studied Microbe: Bacteroides fragilis str. 3397 T10
Show/Hide Hierarchy
|
[4] | |||
| Hierarchy | ||||
| Experimental Method | High-throughput screening | |||
| Description | Colchicine can be metabolized by Bacteroides fragilis str. 3397 T10 (log2FC = -0.533; p = 0.024). | |||
|
Studied Microbe: Bacteroides fragilis str. 3986 T(B)9
Show/Hide Hierarchy
|
[4] | |||
| Hierarchy | ||||
| Experimental Method | High-throughput screening | |||
| Description | Colchicine can be metabolized by Bacteroides fragilis str. 3986 T(B)9 (log2FC = -0.495; p = 0.033). | |||
|
Studied Microbe: Bacteroides fragilis str. DS-208
Show/Hide Hierarchy
|
[4] | |||
| Hierarchy | ||||
| Experimental Method | High-throughput screening | |||
| Description | Colchicine can be metabolized by Bacteroides fragilis str. DS-208 (log2FC = -0.459; p = 0.004). | |||
|
Studied Microbe: Bacteroides uniformis ATCC 8492
Show/Hide Hierarchy
|
[4] | |||
| Hierarchy | ||||
| Experimental Method | High-throughput screening | |||
| Description | Colchicine can be metabolized by Bacteroides uniformis ATCC 8492 (log2FC = -0.56; p = 0.004). | |||
|
Studied Microbe: Bacteroides vulgatus ATCC 8482
Show/Hide Hierarchy
|
[4] | |||
| Hierarchy | ||||
| Experimental Method | High-throughput screening | |||
| Description | Colchicine can be metabolized by Bacteroides vulgatus ATCC 8482 (log2FC = -0.559; p = 0.028). | |||
|
Studied Microbe: Odoribacter splanchnicus
Show/Hide Hierarchy
|
[4] | |||
| Hierarchy | ||||
| Experimental Method | High-throughput screening | |||
| Description | Colchicine can be metabolized by Odoribacter splanchnicus (log2FC = -0.359; p = 0.021). | |||
|
Studied Microbe: Parabacteroides johnsonii DSM 18315
Show/Hide Hierarchy
|
[4] | |||
| Hierarchy | ||||
| Experimental Method | High-throughput screening | |||
| Description | Colchicine can be metabolized by Parabacteroides johnsonii DSM 18315 (log2FC = -0.365; p = 0.048). | |||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Eubacteriales | ||||
|
Studied Microbe: Blautia hansenii DSM20583
Show/Hide Hierarchy
|
[4] | |||
| Hierarchy | ||||
| Experimental Method | High-throughput screening | |||
| Description | Colchicine can be metabolized by Blautia hansenii DSM20583 (log2FC = -0.371; p = 0.002). | |||
|
Studied Microbe: Clostridium sp.
Show/Hide Hierarchy
|
[4] | |||
| Hierarchy | ||||
| Experimental Method | High-throughput screening | |||
| Description | Colchicine can be metabolized by Clostridium sp. (log2FC = -0.408; p = 0.033). | |||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Tubulin (TUB) | Target Info | Binder | [5] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7526). | |||
| REF 2 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||
| REF 3 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (ANDA) 084279. | |||
| REF 4 | Mapping human microbiome drug metabolism by gut bacteria and their genes. Nature. 2019 Jun;570(7762):462-467. | |||
| REF 5 | Vitamin K3 disrupts the microtubule networks by binding to tubulin: a novel mechanism of its antiproliferative activity. Biochemistry. 2009 Jul 28;48(29):6963-74. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

