Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D08BXT
|
|||
| Former ID |
DNC003470
|
|||
| Drug Name |
Spermidine
|
|||
| Synonyms |
Spermidin; UNII-U87FK77H25; BRN 1698591; AI3-26636; EINECS 204-689-0; CHEMBL19612; CHEBI:16610; ATHGHQPFGPMSJY-UHFFFAOYSA-N; U87FK77H25; MFCD00008229; Spermidine hydrochloride; NSC528399; 1pot; Aminopropylbutandiamine; N-(4-Aminobutyl)-1,3-diaminopropane; Spectrum_000005; Tocris-0959; ACMC-20ajn3; AC1L1AQB; Spectrum2_000874; Spectrum3_000977; Spectrum4_001101; Spectrum5_001561; Lopac-S-2501; Biomol-NT_000212; bmse000116; bmse000951; bmse000955; Spermidine 0.1 M solution; Lopac0_001047; SCHEMBL15618; BSPBio_002613; KBioGR_001542; KBioSS_000345; 4-04-00-01300 (Beilstein Handbook Reference); DivK1c_001007; SPBio_000947; Spermidine, > =99% (GC); Spermidine, analytical standard; BPBio1_001276; GTPL2390; DTXSID4036645; CTK3J1693; KBio1_001007; KBio2_000345; KBio2_002913; KBio2_005481; KBio3_001833; MolPort-001-761-230; NINDS_001007; HY-B1776; ZINC1532612; BDBM50009353; PA(34); N-(3-Aminopropyl)-4-aminobutylamine; AKOS006222987; CCG-205124; DB03566; MCULE-8096530192; RTR-003757; SDCCGMLS-0066822.P001; IDI1_001007; NCGC00015937-01; NCGC00015937-02; NCGC00015937-03; NCGC00015937-04; NCGC00015937-05; NCGC00024903-01; NCGC00024903-02; NCGC00024903-03; AJ-26792; AN-22947; LS-45643; M923; NCI60_004294; SC-69371; DB-026892; TR-003757; CS-0013804; FT-0629162; ST24048721; ST45025991; C00315; 124S209; SR0
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Plaque psoriasis [ICD-11: EA90.0; ICD-10: L40.0] | Phase 3 | [1], [2] | |
| Rheumatoid arthritis [ICD-11: FA20] | Phase 3 | [1] | ||
| Structure |
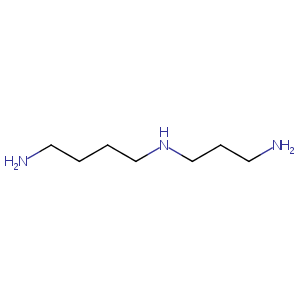 |
Download2D MOL |
||
| Formula |
C7H19N3
|
|||
| Canonical SMILES |
C(CCNCCCN)CN
|
|||
| InChI |
1S/C7H19N3/c8-4-1-2-6-10-7-3-5-9/h10H,1-9H2
|
|||
| InChIKey |
ATHGHQPFGPMSJY-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 124-20-9
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
3609, 600884, 825208, 829689, 833127, 3134393, 5612033, 7890553, 8143482, 8150974, 11111787, 11113819, 11336182, 11361421, 11362759, 11365321, 11367883, 11371932, 11374680, 11376045, 11408685, 11462393, 11484683, 11488690, 11490792, 11492915, 11493819, 11537848, 15146776, 17436308, 24439453, 24771118, 24845842, 24888388, 24888389, 24899434, 24899529, 24899590, 26706955, 26710067, 26713681, 26737363, 26751992, 31647434, 46392889, 46504199, 46504202, 46506086, 47365289, 47440358
|
|||
| ChEBI ID |
CHEBI:16610
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Cytoplasmic thioredoxin reductase (TXNRD1) | Target Info | Inhibitor | [3] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 2 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 3 | How many drug targets are there Nat Rev Drug Discov. 2006 Dec;5(12):993-6. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

