Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D01ZLU
|
|||
| Former ID |
DIB010879
|
|||
| Drug Name |
Futibatinib
|
|||
| Synonyms |
TAS-120
Click to Show/Hide
|
|||
| Indication | Intrahepatic cholangiocarcinoma [ICD-11: 2C12.10; ICD-10: C22.1; ICD-9: 155.1, 156.1] | Approved | [1] | |
| Cholangiocarcinoma [ICD-11: 2C12.10; ICD-10: C22.1] | Phase 3 | [2] | ||
| Multiple myeloma [ICD-11: 2A83; ICD-10: C90.0] | Phase 1/2 | [3] | ||
| Solid tumour/cancer [ICD-11: 2A00-2F9Z] | Phase 1/2 | [4] | ||
| Company |
Taiho oncology
|
|||
| Structure |
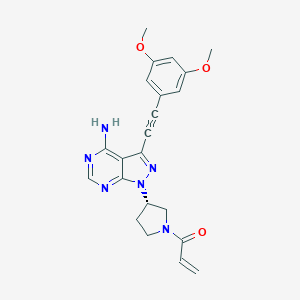 |
Download2D MOL |
||
| Formula |
C22H22N6O3
|
|||
| Canonical SMILES |
COC1=CC(=CC(=C1)C#CC2=NN(C3=NC=NC(=C23)N)C4CCN(C4)C(=O)C=C)OC
|
|||
| InChI |
1S/C22H22N6O3/c1-4-19(29)27-8-7-15(12-27)28-22-20(21(23)24-13-25-22)18(26-28)6-5-14-9-16(30-2)11-17(10-14)31-3/h4,9-11,13,15H,1,7-8,12H2,2-3H3,(H2,23,24,25)/t15-/m0/s1
|
|||
| InChIKey |
KEIPNCCJPRMIAX-HNNXBMFYSA-N
|
|||
| CAS Number |
CAS 1448169-71-8
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Fibroblast growth factor receptor (FGFR) | Target Info | Antagonist | [5] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | FDA Approved Drug Products from FDA Official Website. 2022. Application Number: 214801. | |||
| REF 2 | ClinicalTrials.gov (NCT04093362) Futibatinib Versus Gemcitabine-Cisplatin Chemotherapy as First-Line Treatment of Patients With Advanced Cholangiocarcinoma Harboring FGFR2 Gene Rearrangements (FOENIX-CCA3). U.S. National Institutes of Health. | |||
| REF 3 | ClinicalTrials.gov (NCT02052778) A Dose Finding Study Followed by a Safety and Efficacy Study in Patients With Advanced Solid Tumors or Multiple Myeloma With FGF/FGFR-Related Abnormalities. U.S. National Institutes of Health. | |||
| REF 4 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 5 | TAS-120, a highly potent and selective irreversible FGFR inhibitor, is effective in tumors harboring various FGFR gene abnormalities. Molecular Cancer Therapeutics. 01/2014; 12(11_Supplement):A270-A270. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

