Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D01QXY
|
|||
| Former ID |
DNCL002282
|
|||
| Drug Name |
B7-2/GM-CSF
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Dementia [ICD-11: 6D80-6D86] | Phase 1 | [1] | |
| Dyslipidemia [ICD-11: 5C80-5C81; ICD-9: 272] | Phase 1 | [2], [3] | ||
| Schizophrenia [ICD-11: 6A20] | Phase 1 | [1] | ||
| Solid tumour/cancer [ICD-11: 2A00-2F9Z; ICD-10: C76-C80; ICD-9: 140-229] | Phase 1 | [4] | ||
| Company |
NuVax Therapeutics
|
|||
| Structure |
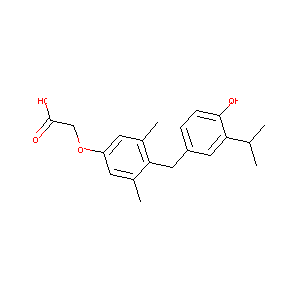 |
Download2D MOL |
||
| Formula |
C20H24O4
|
|||
| Canonical SMILES |
CC1=CC(=CC(=C1CC2=CC(=C(C=C2)O)C(C)C)C)OCC(=O)O
|
|||
| InChI |
1S/C20H24O4/c1-12(2)17-9-15(5-6-19(17)21)10-18-13(3)7-16(8-14(18)4)24-11-20(22)23/h5-9,12,21H,10-11H2,1-4H3,(H,22,23)
|
|||
| InChIKey |
QNAZTOHXCZPOSA-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 211110-63-3
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
14826555, 17396609, 24154997, 45284526, 79888616, 84975215, 85182421, 92098493, 92708720, 96026061, 99443896, 103023065, 103023068, 103327605, 104009556, 123055319, 127346294, 129046650, 134339039, 134340044, 135261431, 135650297, 137468709, 160647443, 160968504, 163620816, 172919515, 184824208, 198942875, 208012256, 223445157, 226626525, 241377054, 249691224, 252158166, 252481017
|
|||
| ChEBI ID |
CHEBI:79988
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Muscarinic acetylcholine receptor M1 (CHRM1) | Target Info | Agonist | [5] |
| Thyroid hormone receptor (THR) | Target Info | Agonist | [6] | |
| KEGG Pathway | Calcium signaling pathway | |||
| cAMP signaling pathway | ||||
| Neuroactive ligand-receptor interaction | ||||
| PI3K-Akt signaling pathway | ||||
| Cholinergic synapse | ||||
| Regulation of actin cytoskeleton | ||||
| Panther Pathway | Alzheimer disease-amyloid secretase pathway | |||
| Heterotrimeric G-protein signaling pathway-Gq alpha and Go alpha mediated pathway | ||||
| Muscarinic acetylcholine receptor 1 and 3 signaling pathway | ||||
| Reactome | Muscarinic acetylcholine receptors | |||
| G alpha (q) signalling events | ||||
| WikiPathways | Monoamine GPCRs | |||
| Calcium Regulation in the Cardiac Cell | ||||
| Regulation of Actin Cytoskeleton | ||||
| GPCRs, Class A Rhodopsin-like | ||||
| Gastrin-CREB signalling pathway via PKC and MAPK | ||||
| Secretion of Hydrochloric Acid in Parietal Cells | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800028844) | |||
| REF 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2639). | |||
| REF 3 | ClinicalTrials.gov (NCT01787578) Safety and Pharmacodynamic Study of Sobetirome in X-Linked Adrenoleukodystrophy (X-ALD). U.S. National Institutes of Health. | |||
| REF 4 | GM-CSF-Secreting Vaccines for Solid Tumors: Moving Forward. Discov Med. 2010 July; 10(50): 52-60. | |||
| REF 5 | Clinical pipeline report, company report or official report of GlaxoSmithKline (2009). | |||
| REF 6 | Sobetirome: a case history of bench-to-clinic drug discovery and development. Heart Fail Rev. 2010 Mar;15(2):177-82. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

