Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D01QUS
|
|||
| Former ID |
DAP000292
|
|||
| Drug Name |
Calcipotriol
|
|||
| Synonyms |
Calcipotriene; Divonex; Dovonex; BMS-181161; Daivonex (TN); Dovonex (TN); MC-903; (1R,3S,5Z)-5-[(2E)-2-[(1R,3aS,7aR)-1-[(E,2R,5S)-5-cyclopropyl-5-hydroxypent-3-en-2-yl]-7a-methyl-2,3,3a,5,6,7-hexahydro-1H-inden-4-ylidene]ethylidene]-4-methylidenecyclohexane-1,3-diol; (1S,5Z,7Z,17ALPHA,22E)-24-CYCLOPROPYL-9,10-SECOCHOLA-5,7,10,22-TETRAENE-1,3,24-TRIOL; 1-ALPHA,24S-(OH)2-22-ENE-26,27-DEHYDROVITAMIN D3
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Psoriasis vulgaris [ICD-11: EA90; ICD-9: 696] | Approved | [1], [2] | |
| Therapeutic Class |
Antipsoriatic Agents
|
|||
| Company |
LEO Pharma Inc
|
|||
| Structure |
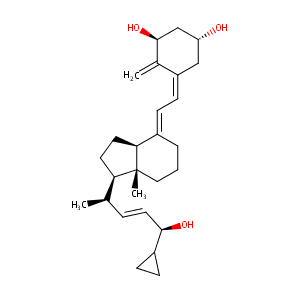 |
Download2D MOL |
||
| Formula |
C27H40O3
|
|||
| Canonical SMILES |
CC(C=CC(C1CC1)O)C2CCC3C2(CCCC3=CC=C4CC(CC(C4=C)O)O)C
|
|||
| InChI |
1S/C27H40O3/c1-17(6-13-25(29)20-8-9-20)23-11-12-24-19(5-4-14-27(23,24)3)7-10-21-15-22(28)16-26(30)18(21)2/h6-7,10,13,17,20,22-26,28-30H,2,4-5,8-9,11-12,14-16H2,1,3H3/b13-6+,19-7+,21-10-/t17-,22-,23-,24+,25-,26+,27-/m1/s1
|
|||
| InChIKey |
LWQQLNNNIPYSNX-UROSTWAQSA-N
|
|||
| CAS Number |
CAS 112965-21-6
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
7848188, 7888845, 7978843, 11041473, 12014175, 14715113, 14855474, 14855477, 26719860, 39321118, 46386727, 46507122, 49681738, 50112719, 53789506, 56352848, 57359242, 71824952, 74547909, 92308501, 92308864, 103770649, 113872806, 124658943, 127310141, 127310142, 127310143, 127310144, 127310145, 127310146, 127310147, 127310148, 127310149, 127310150, 127310151, 135028596, 135650047, 135698205, 137156387, 139629435, 143493294, 143493295, 144206022, 151994658, 152164586, 152237515, 152238027, 152258045, 160646883, 160849066
|
|||
| ChEBI ID |
CHEBI:50749
|
|||
| ADReCS Drug ID | BADD_D00334 | |||
| SuperDrug ATC ID |
D05AX02
|
|||
| SuperDrug CAS ID |
cas=112965216
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Vitamin D3 receptor (VDR) | Target Info | Antagonist | [3] |
| KEGG Pathway | Endocrine and other factor-regulated calcium reabsorption | |||
| Mineral absorption | ||||
| Tuberculosis | ||||
| NetPath Pathway | IL4 Signaling Pathway | |||
| Panther Pathway | Vitamin D metabolism and pathway | |||
| Pathway Interaction Database | Regulation of nuclear SMAD2/3 signaling | |||
| Direct p53 effectors | ||||
| RXR and RAR heterodimerization with other nuclear receptor | ||||
| Retinoic acid receptors-mediated signaling | ||||
| Validated transcriptional targets of deltaNp63 isoforms | ||||
| Validated transcriptional targets of TAp63 isoforms | ||||
| Reactome | Nuclear Receptor transcription pathway | |||
| WikiPathways | Ovarian Infertility Genes | |||
| Nuclear Receptors in Lipid Metabolism and Toxicity | ||||
| Nuclear Receptors Meta-Pathway | ||||
| Vitamin D Receptor Pathway | ||||
| Drug Induction of Bile Acid Pathway | ||||
| Nuclear Receptors | ||||
| Vitamin D Metabolism | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2778). | |||
| REF 2 | Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007 Mar;70(3):461-77. | |||
| REF 3 | Cellular and molecular mechanisms involved in the action of vitamin D analogs targeting vitiligo depigmentation. Curr Drug Targets. 2008 Apr;9(4):345-59. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

