Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D01HST
|
|||
| Former ID |
DIB018833
|
|||
| Drug Name |
amg-1
|
|||
| Synonyms |
CHEMBL3342402; N-(1,3-benzodioxol-5-yl)-2-{[5-(4-methylphenyl)[1,3]thiazolo[2,3-c][1,2,4]triazol-3-yl]sulfanyl}acetamide; BAS 06591295; AC1LLIU7; GTPL8227; SCHEMBL20313457; MolPort-000-220-791; ZINC801901; STL305655; BDBM50030791; AKOS000657540; MCULE-5294683561; ST50049773; Z56882059; N-(2H-1,3-benzodioxol-5-yl)-2-{[5-(4-methylphenyl)-[1,2,4]triazolo[3,4-b][1,3]thiazol-3-yl]sulfanyl}acetamide; 496023-55-3; N-(2H-benzo[3,4-d]1,3-dioxolen-5-yl)-2-[5-(4-methylphenyl)(1,3-thiazolino[3,2- d]1,2,4-triazol-3-ylthio)]acetamide
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Discovery agent [ICD-11: N.A.] | Investigative | [1] | |
| Structure |
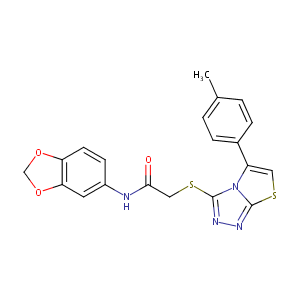 |
Download2D MOL
|
||
| Formula |
C20H16N4O3S2
|
|||
| Canonical SMILES |
CC1=CC=C(C=C1)C2=CSC3=NN=C(N23)SCC(=O)NC4=CC5=C(C=C4)OCO5
|
|||
| InChI |
1S/C20H16N4O3S2/c1-12-2-4-13(5-3-12)15-9-28-19-22-23-20(24(15)19)29-10-18(25)21-14-6-7-16-17(8-14)27-11-26-16/h2-9H,10-11H2,1H3,(H,21,25)
|
|||
| InChIKey |
XYXNYQAJSYUYNZ-UHFFFAOYSA-N
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Indoleamine 2,3-dioxygenase 1 (IDO1) | Target Info | Inhibitor | [1] |
| BioCyc | Superpathway of tryptophan utilization | |||
| Tryptophan degradation | ||||
| L-kynurenine degradation | ||||
| Tryptophan degradation to 2-amino-3-carboxymuconate semialdehyde | ||||
| NAD de novo biosynthesis | ||||
| KEGG Pathway | Tryptophan metabolism | |||
| Metabolic pathways | ||||
| African trypanosomiasis | ||||
| NetPath Pathway | TSLP Signaling Pathway | |||
| IL5 Signaling Pathway | ||||
| TGF_beta_Receptor Signaling Pathway | ||||
| Pathwhiz Pathway | Tryptophan Metabolism | |||
| Reactome | Tryptophan catabolism | |||
| WikiPathways | Tryptophan metabolism | |||
| Metabolism of amino acids and derivatives | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Purification and kinetic characterization of human indoleamine 2,3-dioxygenases 1 and 2 (IDO1 and IDO2) and discovery of selective IDO1 inhibitors. Biochim Biophys Acta. 2011 Dec;1814(12):1947-54. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

