Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D00QWU
|
|||
| Former ID |
DNCL001803
|
|||
| Drug Name |
BMS-779788
|
|||
| Indication | Arteriosclerosis [ICD-11: BD40; ICD-10: I70] | Phase 1 | [1] | |
| Company |
Bristol-Myers Squibb; Exelixis
|
|||
| Structure |
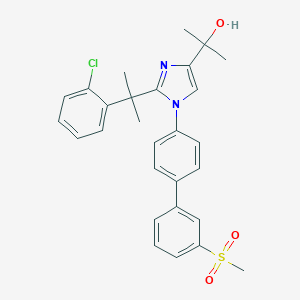 |
Download2D MOL |
||
| Formula |
C28H29ClN2O3S
|
|||
| Canonical SMILES |
CC(C)(C1=CC=CC=C1Cl)C2=NC(=CN2C3=CC=C(C=C3)C4=CC(=CC=C4)S(=O)(=O)C)C(C)(C)O
|
|||
| InChI |
1S/C28H29ClN2O3S/c1-27(2,23-11-6-7-12-24(23)29)26-30-25(28(3,4)32)18-31(26)21-15-13-19(14-16-21)20-9-8-10-22(17-20)35(5,33)34/h6-18,32H,1-5H3
|
|||
| InChIKey |
JLPURTXCSILYLW-UHFFFAOYSA-N
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Oxysterols receptor LXR (NR1H) | Target Info | Modulator | [2] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT00836602) Multiple-Dose Study to Evaluate the Safety, Pharmacokinetics and Pharmacodynamics of BMS-779788 in Healthy Subjects. U.S. National Institutes of Health. | |||
| REF 2 | Liver X receptors in lipid metabolism: opportunities for drug discovery. Nat Rev Drug Discov. 2014 Jun;13(6):433-44. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

