Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D00ACX
|
|||
| Former ID |
DNC003265
|
|||
| Drug Name |
2-chloro-5-nitro-N-phenylbenzamide
|
|||
| Synonyms |
2-Chloro-5-nitro-N-phenylbenzamide; GW9662; 22978-25-2; GW 9662; 2-Chloro-5-nitrobenzanilide; GW-9662; MLS001056751; CHEBI:79993; 2-Chloro-5-nitro-N-phenyl-benzamide; 2-Chloro-5-nitro-N-4-phenylbenzamide; benzamide, 2-chloro-5-nitro-N-phenyl-; SMR000326735; (2-chloro-5-nitrophenyl)-N-benzamide; SR-01000075999; Tocris-1508; Spectrum5_001989; Lopac-M-6191; AC1LD8S0; DSSTox_RID_79570; DSSTox_CID_20723; DSSTox_GSID_40723; Lopac0_000798; KBioGR_000361; KBioSS_000361; BSPBio_001021; SCHEMBL420231; CHEMBL375270; GTPL3442; cid_644213
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Discovery agent [ICD-11: N.A.] | Investigative | [1], [2] | |
| Structure |
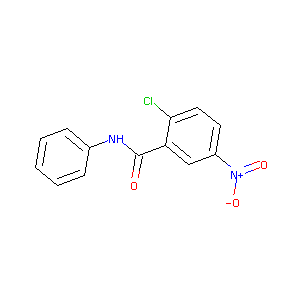 |
Download2D MOL |
||
| Formula |
C13H9ClN2O3
|
|||
| Canonical SMILES |
C1=CC=C(C=C1)NC(=O)C2=C(C=CC(=C2)[N+](=O)[O-])Cl
|
|||
| InChI |
1S/C13H9ClN2O3/c14-12-7-6-10(16(18)19)8-11(12)13(17)15-9-4-2-1-3-5-9/h1-8H,(H,15,17)
|
|||
| InChIKey |
DNTSIBUQMRRYIU-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 22978-25-2
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
841875, 877487, 6873016, 8707317, 11111477, 11114114, 11121686, 11122166, 11362775, 11365337, 11367899, 11370801, 11370802, 11373500, 11376061, 14824325, 17396617, 17405359, 22395870, 24278560, 24883187, 26732595, 26752209, 26752210, 26752211, 26758841, 43765920, 47719268, 47793670, 47942452, 47942453, 48392499, 50104878, 50104879, 50104880, 53777904, 53790960, 53800743, 56355093, 57408227, 77530674, 85202868, 85231138, 85787762, 88369062, 90341221, 92304217, 92310037, 99300949, 99302469
|
|||
| ChEBI ID |
CHEBI:79993
|
|||
| Interaction between the Drug and Microbe | Top | |||
|---|---|---|---|---|
| The Metabolism of Drug Affected by Studied Microbe(s) | ||||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Gut microbiota | ||||
| Studied Microbe: Gut microbiota unspecific | [3], [4] | |||
| Microbial Enzyme | Nitroreductase | |||
| Metabolic Reaction | Nitroreduction | |||
| Description | 2-chloro-5-nitro-N-phenylbenzamide can be metabolized by the nitroreductase of gut microbiota through nitroreduction. | |||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | The Protein Data Bank. Nucleic Acids Res. 2000 Jan 1;28(1):235-42. | |||
| REF 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 3442). | |||
| REF 3 | Drug Metabolism by the Host and Gut Microbiota: A Partnership or Rivalry?. Drug Metab Dispos. 2015 Oct;43(10):1499-504. | |||
| REF 4 | Effects of bacterial and presystemic nitroreductase metabolism of 2-chloro-5-nitro-N-phenylbenzamide on its mutagenicity and bioavailability. Chem Biol Interact. 2012 Apr 15;197(1):16-22. | |||
| REF 5 | Tryptophan-containing dipeptide derivatives as potent PPARgamma antagonists: design, synthesis, biological evaluation, and molecular modeling. Eur J Med Chem. 2008 Dec;43(12):2699-716. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

