| Drug General Information |
| Drug ID |
D0BP9C
|
| Drug Name |
Etravirine |
|
| Synonyms |
Intelence; DAPY deriv; Diaminopyrimidine deriv; R 165335; R165335; TMC 125; TMC125; Intelence (TN); Intelence(TM); R-165335; R165335-TMC125; TMC-125; Etravirine (JAN/USAN/INN); TMC-125/R-165335; 4-((6-amino-5-bromo-2-((4-cyanophenyl)amino)-4-pyrimidinyl)oxy)-3,5-dimethyl-benzonitrile; 4-({6-AMINO-5-BROMO-2-[(4-CYANOPHENYL)AMINO]PYRIMIDIN-4-YL}OXY)-3,5-DIMETHYLBENZONITRILE; 4-[6-amino-5-bromo-2-(4-cyanoanilino)pyrimidin-4-yl]oxy-3,5-dimethylbenzonitrile; 65B; ETR |
| Drug Type |
Small molecular drug |
| Structure |
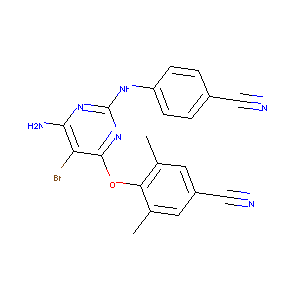
|
| Drug Resistance Mutations |
| Target Name |
HIV Non-Nucleoside reverse transcriptase |
Target Info |
| Uniprot ID |
POL_HV1B1(600-1159) |
| Species |
Human immunodeficiency virus type 1 (HIV-1) |
| Reference Sequence |
PISPIETVPVKLKPGMDGPKVKQWPLTEEKIKALVEICTEMEKEGKISKIGPENPYNTPV
FAIKKKDSTKWRKLVDFRELNKRTQDFWEVQLGIPHPAGLKKKKSVTVLDVGDAYFSVPL
DEDFRKYTAFTIPSINNETPGIRYQYNVLPQGWKGSPAIFQSSMTKILEPFKKQNPDIVI
YQYMDDLYVGSDLEIGQHRTKIEELRQHLLRWGLTTPDKKHQKEPPFLWMGYELHPDKWT
VQPIVLPEKDSWTVNDIQKLVGKLNWASQIYPGIKVRQLCKLLRGTKALTEVIPLTEEAE
LELAENREILKEPVHGVYYDPSKDLIAEIQKQGQGQWTYQIYQEPFKNLKTGKYARMRGA
HTNDVKQLTEAVQKITTESIVIWGKTPKFKLPIQKETWETWWTEYWQATWIPEWEFVNTP
PLVKLWYQLEKEPIVGAETFYVDGAANRETKLGKAGYVTNKGRQKVVPLTNTTNQKTELQ
AIYLALQDSGLEVNIVTDSQYALGIIQAQPDKSESELVNQIIEQLIKKEKVYLAWVPAHK
GIGGNEQVDKLVSAGIRKIL [Human immunodeficiency virus type 1 (H
IV-1)]
|
| Targeted Disease |
HIV infection |
| Drug Resistance Mutations |
| Mutation info |
Missense: Y188L |
[1] |
| Level of Resistance |
Reduce susceptibility to ETR |
|
| Mutation info |
Missense: K101P |
[2] |
| Level of Resistance |
Reduce ETR susceptibility 5 fold |
|
| Mutation info |
Missense: L100I |
[3] |
| Level of Resistance |
Intermediate resistance |
|
| Mutation info |
Missense: Y181C |
[2] |
| Level of Resistance |
Reduce ETR susceptibility 5 fold |
|
| Mutation info |
Missense: E138K |
[4], [5], [6] |
| Level of Resistance |
Reduce ETR susceptibility about 2 fold |
|
| Mutation info |
Missense: K101E |
[2], [3], [4] |
| Level of Resistance |
Reduce ETR susceptibility 1-5 fold |
|
| Mutation info |
Missense: G190E |
[7], [8], [9] |
| Level of Resistance |
Confer high-level resistance to ETR |
|
| Mutation info |
Missense: V179E |
[9], [10], [11] |
| Level of Resistance |
Confer low-level resistance to ETR |
|
| Mutation info |
Missense: K238T |
[9], [10], [12] |
| Level of Resistance |
Reduce susceptibility to ETR |
|
| Mutation info |
Missense: G190Q |
[13], [14] |
| Level of Resistance |
Reduce susceptibility to ETR |
|
| Mutation info |
Missense: V179D |
[11], [14] |
| Level of Resistance |
Reduce ETR susceptibility 2-3 fold |
|
| Mutation info |
Missense: K238N |
[15], [9] |
| Level of Resistance |
Reduce susceptibility to ETR |
|
| Mutation info |
Missense: V179F |
[4], [16] |
| Level of Resistance |
Low-level resistance |
|
| Mutation info |
Missense: L100V |
[17], [9] |
| Level of Resistance |
Reduce susceptibility to ETR |
|
| Mutation info |
Missense: E138G |
[18], [19] |
|
| Mutation info |
Missense: E138Q |
[18], [19] |
|
| Mutation info |
Missense: G190A |
[18], [19] |
|
| Mutation info |
Missense: G190S |
[18], [19] |
|
| Mutation info |
Missense: M230L |
[18], [19] |
| Level of Resistance |
Intermediate resistance |
|
| Mutation info |
Missense: V179F + Y181C |
[18], [19] |
| Level of Resistance |
Low-level resistance |
|
| Mutation info |
Missense: Y181F |
[18], [19] |
| Level of Resistance |
Low-level resistance |
|
| Mutation info |
Missense: Y181G |
[18], [19] |
| Level of Resistance |
Low-level resistance |
|
| Mutation info |
Missense: Y181I |
[18], [19] |
| Level of Resistance |
High-level resistance |
|
| Mutation info |
Missense: Y181S |
[18], [19] |
| Level of Resistance |
Low-level resistance |
|
| Mutation info |
Missense: Y181V |
[18], [19] |
| Level of Resistance |
High-level resistance |
|
| Mutation info |
Missense: E138A |
[5] |
| Level of Resistance |
Reduce ETR susceptibility about 2 fold |
|
| Mutation info |
Missense: E138R |
[20] |
| Level of Resistance |
Reduce ETR susceptibility 2-3 fold |
|
| Mutation info |
Missense: F227C |
[20] |
| Level of Resistance |
Reduce ETR susceptibility 4-5 fold |
|
| Mutation info |
Missense: M230I |
[20] |
| Level of Resistance |
Reduce ETR susceptibility 9 fold |
|
| References |
| REF 1 |
Genotypic correlates of phenotypic resistance to efavirenz in virus isolates from patients failing nonnucleoside reverse transcriptase inhibitor therapy. J Virol. 2001 Jun;75(11):4999-5008.
|
| REF 2 |
Constrained patterns of covariation and clustering of HIV-1 non-nucleoside reverse transcriptase inhibitor resistance mutations. J Antimicrob Chemother. 2010 Jul;65(7):1477-85.
|
| REF 3 |
Human immunodeficiency virus type 1 mutations selected in patients failing efavirenz combination therapy. Antimicrob Agents Chemother. 2000 Sep;44(9):2475-84.
|
| REF 4 |
Characterization of genotypic and phenotypic changes in HIV-1-infected patients with virologic failure on an etravirine-containing regimen in the DUET-1 and DUET-2 clinical studies. AIDS Res Hum Retroviruses. 2010 Nov;26(11):1197-205.
|
| REF 5 |
Effect of mutations at position E138 in HIV-1 reverse transcriptase on phenotypic susceptibility and virologic response to etravirine. J Acquir Immune Defic Syndr. 2011 Sep 1;58(1):18-22.
|
| REF 6 |
Genotypic and phenotypic characterization of HIV-1 isolates obtained from patients on rilpivirine therapy experiencing virologic failure in the phase 3 ECHO and THRIVE studies: 48-week analysis. J Acquir Immune Defic Syndr. 2012 Jan 1;59(1):39-46.
|
| REF 7 |
TMC125 displays a high genetic barrier to the development of resistance: evidence from in vitro selection experiments. J Virol. 2005 Oct;79(20):12773-82.
|
| REF 8 |
TMC278, a next-generation nonnucleoside reverse transcriptase inhibitor (NNRTI), active against wild-type and NNRTI-resistant HIV-1. Antimicrob Agents Chemother. 2010 Feb;54(2):718-27.
|
| REF 9 |
Significantly improved HIV inhibitor efficacy prediction employing proteochemometric models generated from antivirogram data. PLoS Comput Biol. 2013;9(2):e1002899.
|
| REF 10 |
Human immunodeficiency virus reverse transcriptase and protease sequence database. Nucleic Acids Res. 2003 Jan 1;31(1):298-303.
|
| REF 11 |
Compilation and prevalence of mutations associated with resistance to non-nucleoside reverse transcriptase inhibitors. Antivir Ther. 2009;14(1):103-9.
|
| REF 12 |
The K101P and K103R/V179D mutations in human immunodeficiency virus type 1 reverse transcriptase confer resistance to nonnucleoside reverse transcriptase inhibitors. Antimicrob Agents Chemother. 2006 Jan;50(1):351-4.
|
| REF 13 |
Amino acid substitutions at position 190 of human immunodeficiency virus type 1 reverse transcriptase increase susceptibility to delavirdine and impair virus replication. J Virol. 2003 Jan;77(2):1512-23.
|
| REF 14 |
Non-nucleoside reverse transcriptase inhibitor (NNRTI) cross-resistance: implications for preclinical evaluation of novel NNRTIs and clinical genotypic resistance testing. J Antimicrob Chemother. 2014 Jan;69(1):12-20.
|
| REF 15 |
Nonpolymorphic human immunodeficiency virus type 1 protease and reverse transcriptase treatment-selected mutations. Antimicrob Agents Chemother. 2009 Nov;53(11):4869-78.
|
| REF 16 |
Emerging mutations and associated factors in patients displaying treatment failure on an etravirine-containing regimen. Antivir Ther. 2012;17(1):119-23.
|
| REF 17 |
Quantitative prediction of integrase inhibitor resistance from genotype through consensus linear regression modeling. Virol J. 2013 Jan 3;10:8.
|
| REF 18 |
The HIVdb system for HIV-1 genotypic resistance interpretation. Intervirology. 2012;55(2):98-101.
|
| REF 19 |
HIV-1 drug resistance and resistance testing. Infect Genet Evol. 2016 Dec;46:292-307.
|
| REF 20 |
Impact of drug resistance-associated amino acid changes in HIV-1 subtype C on susceptibility to newer nonnucleoside reverse transcriptase inhibitors. Antimicrob Agents Chemother. 2015 Feb;59(2):960-71.
|


