Resistance mutation info of drug
| Drug General Information | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drug ID | D07TQV | |||||||||||||||
| Drug Name | Lamivudine | |||||||||||||||
| Synonyms | lamivudine; 134678-17-4; Epivir; Zeffix; Heptovir; Epivir-HBV; Hepitec; Heptodin; BCH-189; 3TC; Heptivir; CIS-LAMIVUDINE; (-)-2'-Deoxy-3'-thiacytidine; 4-amino-1-((2R,5S)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl)pyrimidin-2(1H)-one; GR-109714X; 3'-Thia-2',3'-dideoxycytidine; (-)-BCH-189; Lamivudine [USAN:BAN:INN]; GR109714X; beta-L-3'-Thia-2',3'-dideoxycytidine; beta-L-2',3'-Dideoxy-3'-thiacytidine; (-)NGPB-21; 136891-12-8; 2',3'-Dideoxy-3'-thiacytidine; (-)-BCH 189; UNII-2T8Q726O95; HSDB 7155; GR 109714X; DTHC; LMV; Lamivir; Zefix; BCH 189; BCH189; BCH-790; DRG-0126; Epivir (TN); Epivir(TM); GG-714; HHA & 3TC; HHA & Lamivudine; Heptovir (TN); Lamivudine & GNA; Zeffix (TN); Epivir-HBV (TN); Lamivudine [USAN:INN:BAN]; Lamivudine (JAN/USP/INN); Lamivudine, (2S-cis)-Isomer; Beta-L-2',3'-Dideoxy-3'-thiacytidine; Beta-L-3'-Thia-2',3'-dideoxycytidine; Beta-L-(-)-2',3'-dideoxy-3'-thiacytidine & Sho-Saiko-To; (+/-)-(Cis)-1-[2-(Hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine; (+/-)-3TC; (+/-)-BCH-189; (+/-)-SddC; (-)-(2'R,5'S)-1-[2'-Hydroxymethyl-5'-(1,3-oxathiolanyl)]cytosine; (-)-1-((2R,5S)-2-(Hydroxymethyl)-1,3-oxathiolan-5-yl)cytosine; (-)-1-[(2R,5S)-2-(Hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine; (-)-NGPB-21; (-)-SddC; (-)-beta-L-2',3'-Dideoxy-3'-thiacytidine; (2R,cis)-4-amino-1-(2-hydroxymethyl-1,3-oxathiolan-5-yl)-(1H)-pyrimidin-2-one; 2',3' Dideoxy 3' thiacytidine; 2(1H)-Pyrimidinone, 4-amino-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl], (+/-)-(Cis); 2(1H)-Pyrimidinone, 4-amino-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl], (-)-(2R,5S); 2(1H)-Pyrimidinone, 4-amino-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl], (-)-(2R,5S) & Galanthus Nivalis Agglutinin (GNA); 2(1H)-Pyrimidinone, 4-amino-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl], (-)-(2R,5S) & Hippeastrum hybrid agglutinin(HHA); 3TC & GNA; 3TC & SST; 3TC (AIDS INITIATIVE) (AIDS INITIATIVE); 3TC and NV-01; 3TC, Zeffix, Heptovir, Epivir, Epivir-HBV, Lamivudine; 4-Amino-1-((2R,5S)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl)-2(1H)-pyrimidinone; 4-amino-1-[(2R,5S)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]pyrimidin-2(1H)-one; 4-amino-1-[(2R,5S)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]pyrimidin-2-one; Efavirenz/lamivudine/tenofovir fumarate | |||||||||||||||
| Drug Type | Small molecular drug | |||||||||||||||
| Therapeutic Class | Anti-HIV Agents | |||||||||||||||
| Company | Glaxo Smith Kline Pharmaceuticals | |||||||||||||||
| Structure |
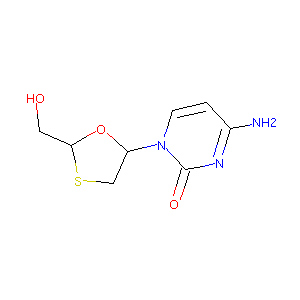
|
|||||||||||||||
| Drug Resistance Mutations | ||||||||||||||||
| Target Name | HIV Nucleoside reverse transcriptase | Target Info | ||||||||||||||
| Uniprot ID | POL_HV1B1(600-1159) | |||||||||||||||
| Species | Human immunodeficiency virus type 1 (HIV-1) | |||||||||||||||
| Reference Sequence |
PISPIETVPVKLKPGMDGPKVKQWPLTEEKIKALVEICTEMEKEGKISKIGPENPYNTPV FAIKKKDSTKWRKLVDFRELNKRTQDFWEVQLGIPHPAGLKKKKSVTVLDVGDAYFSVPL DEDFRKYTAFTIPSINNETPGIRYQYNVLPQGWKGSPAIFQSSMTKILEPFKKQNPDIVI YQYMDDLYVGSDLEIGQHRTKIEELRQHLLRWGLTTPDKKHQKEPPFLWMGYELHPDKWT VQPIVLPEKDSWTVNDIQKLVGKLNWASQIYPGIKVRQLCKLLRGTKALTEVIPLTEEAE LELAENREILKEPVHGVYYDPSKDLIAEIQKQGQGQWTYQIYQEPFKNLKTGKYARMRGA HTNDVKQLTEAVQKITTESIVIWGKTPKFKLPIQKETWETWWTEYWQATWIPEWEFVNTP PLVKLWYQLEKEPIVGAETFYVDGAANRETKLGKAGYVTN [Human immunodefici ency virus type 1 (HIV-1)] |
|||||||||||||||
| Targeted Disease | HIV infection; HBV infection | |||||||||||||||
| Drug Resistance Mutations |
|
|||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
| Target Name | HIV Non-Nucleoside reverse transcriptase | Target Info | ||||||||||||||
| Uniprot ID | POL_HV1B1(600-1159) | |||||||||||||||
| Species | Human immunodeficiency virus type 1 (HIV-1) | |||||||||||||||
| Reference Sequence |
PISPIETVPVKLKPGMDGPKVKQWPLTEEKIKALVEICTEMEKEGKISKIGPENPYNTPV FAIKKKDSTKWRKLVDFRELNKRTQDFWEVQLGIPHPAGLKKKKSVTVLDVGDAYFSVPL DEDFRKYTAFTIPSINNETPGIRYQYNVLPQGWKGSPAIFQSSMTKILEPFKKQNPDIVI YQYMDDLYVGSDLEIGQHRTKIEELRQHLLRWGLTTPDKKHQKEPPFLWMGYELHPDKWT VQPIVLPEKDSWTVNDIQKLVGKLNWASQIYPGIKVRQLCKLLRGTKALTEVIPLTEEAE LELAENREILKEPVHGVYYDPSKDLIAEIQKQGQGQWTYQIYQEPFKNLKTGKYARMRGA HTNDVKQLTEAVQKITTESIVIWGKTPKFKLPIQKETWETWWTEYWQATWIPEWEFVNTP PLVKLWYQLEKEPIVGAETFYVDGAANRETKLGKAGYVTNKGRQKVVPLTNTTNQKTELQ AIYLALQDSGLEVNIVTDSQYALGIIQAQPDKSESELVNQIIEQLIKKEKVYLAWVPAHK GIGGNEQVDKLVSAGIRKIL [Human immunodeficiency virus type 1 (H IV-1)] |
|||||||||||||||
| Targeted Disease | HIV infection; HBV infection | |||||||||||||||
| Drug Resistance Mutations |
|
|||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
| Target Name | HBV Reverse transcriptase | Target Info | ||||||||||||||
| Uniprot ID | DPOL_HBVB5(347-690) | |||||||||||||||
| Species | Hepatitis B virus genotype B (HBV-B) | |||||||||||||||
| Reference Sequence |
EDWGPCTEHGEHRIRTPRTPARVTGGVFLVDKNPHNTTESRLVVDFSQFSRGNTRVSWPK FAVPNLQSLTNLLSSNLSWLSLDVSAAFYHLPLHPAAMPHLLVGSSGLSRYVARLSSNSR IINNQHRTMQNLHNSCSRNLYVSLMLLYKTYGRKLHLYSHPIILGFRKIPMGVGLSPFLL AQFTSAICSVVRRAFPHCLAFSYMDDVVLGAKSVQHLESLYAAVTNFLLSLGIHLNPHKT KRWGYSLNFMGYVIGSWGTLPQEHIVQKIKMCFRKLPVNRPIDWKVCQRIVGLLGFAAPF TQCGYPALMPLYACIQAKQAFTFSPTYKAFLSKQYLNLYPVARQ [Hepatitis B vi rus genotype B (HBV-B)] |
|||||||||||||||
| Targeted Disease | HIV infection; HBV infection | |||||||||||||||
| Drug Resistance Mutations |
|
|||||||||||||||
| References | ||||||||||||||||
| REF 1 | The HIVdb system for HIV-1 genotypic resistance interpretation. Intervirology. 2012;55(2):98-101. | |||||||||||||||
| REF 2 | How to win the HIV-1 drug resistance hurdle race: running faster or jumping higher Biochem J. 2017 Apr 26;474(10):1559-1577. | |||||||||||||||
| REF 3 | Clinical correlates and molecular basis of HIV drug resistance. J Acquir Immune Defic Syndr. 1993;6 Suppl 1:S36-46. | |||||||||||||||
| REF 4 | Mechanisms of anti-retroviral drug resistance: implications for novel drug discovery and development. Infect Disord Drug Targets. 2013 Oct;13(5):330-6. | |||||||||||||||
| REF 5 | Comparison of Detection Rate and Mutational Pattern of Drug-Resistant Mutations Between a Large Cohort of Genotype B and Genotype C Hepatitis B Virus-Infected Patients in North China. Microb Drug Resist. 2017 Jun;23(4):516-522. | |||||||||||||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

