Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D01AQT
|
||||
| Former ID |
DAP000629
|
||||
| Drug Name |
Terconazole
|
||||
| Synonyms |
Fungistat; Terazol; Terconazol; Terconazolum; Tercospor; Triaconazole; Zazole; R 42470; Terazol 3; Terazol 7; Terazol 73; Tetrazol 3; Tetrazol 7; Gyno-Terazol; R 42,470; R-42470; Terazol 3 (TN); Terazol 7 & 3; Terazol Cream & Suppositories; Terconazol [INN-Spanish]; Terconazolum [INN-Latin]; Terconazole (USAN/INN); Terconazole [USAN:INN:BAN]; Cis-1-(p-((2-(2,4-Dichlorophenyl)-2-(1H-1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4-yl)methoxy)phenyl)-4-isopropylpiperazine; Piperazine, 1-[4-[[2-(2,4-dichlorophenyl)-2-(1H-1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy]phenyl]-4-(1-methylethyl)-, rel-(2R,4S); 1-(4-((2-(2,4-dichlorophenyl)-2-(1H-1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4-yl)methoxy)phenyl)-4-(1-methylethyl)piperazine; 1-[4-[[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy]phenyl]-4-propan-2-ylpiperazine; 1-[4-[[(2S,4R)-2-(2,4-dichlorophenyl)-2-(1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy]phenyl]-4-propan-2-ylpiperazine; 1-[4-[[(4S)-2-(2,4-dichlorophenyl)-2-(1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy]phenyl]-4-propan-2-ylpiperazine; 1-[4-[[2-(2,4-dichlorophenyl)-2-(1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy]phenyl]-4-propan-2-ylpiperazine
|
||||
| Drug Type |
Small molecular drug
|
||||
| Therapeutic Class |
Antifungal Agents
|
||||
| Structure |
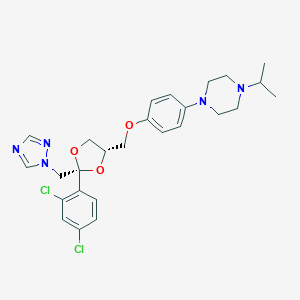
|
Download2D MOL |
|||
| Formula |
C26H31Cl2N5O3
|
||||
| Canonical SMILES |
CC(C)N1CCN(CC1)C2=CC=C(C=C2)OCC3COC(O3)(CN4C=NC=N4)C5=C<br />(C=C(C=C5)Cl)Cl
|
||||
| InChI |
1S/C26H31Cl2N5O3/c1-19(2)31-9-11-32(12-10-31)21-4-6-22(7-5-21)34-14-23-15-35-26(36-23,16-33-18-29-17-30-33)24-8-3-20(27)13-25(24)28/h3-8,13,17-19,23H,9-12,14-16H2,1-2H3/t23?,26-/m0/s1
|
||||
| InChIKey |
BLSQLHNBWJLIBQ-NASUQTAISA-N
|
||||
| CAS Number |
CAS 67915-31-5
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID | |||||
| SuperDrug ATC ID |
G01AG02
|
||||
| SuperDrug CAS ID |
cas=067915315
|
||||
| Target and Pathway | |||||
| Target(s) | Fungal Cytochrome P450 51 | Target Info | Inhibitor | [537454] | |
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

