Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D08HOM
|
|||
| Former ID |
DNCL002384
|
|||
| Drug Name |
ABT-348
|
|||
| Synonyms |
ILORASERTIB; ABT-348; 1227939-82-3; UNII-6L5D03D975; ABT 348; ABT348; 6L5D03D975; A-968660; Abbott-969660; Ilorasertib [USAN:INN]; Ilorasertib (USAN); Kinome_405; GTPL9914; A-968660.0; SCHEMBL3381224; CHEMBL1980297; DTXSID10153718; WPHKIQPVPYJNAX-UHFFFAOYSA-N; BCP07256; ZINC63298074; BDBM50381716; SB16853; CS-6804; DB11694; KB-74395; HY-16018; US8722890, 1; Z-3287; US8722890, 2; D10423; N-(4-{4-amino-7-[1-(2-hydroxyethyl)-1H-pyrazol-4-yl]thieno[3,2-c]pyridin-3-yl}phenyl)-N'-(3-fluorophenyl)urea
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Haematological malignancy [ICD-11: 2B33.Y] | Phase 2 | [1] | |
| Company |
Abbott Laboratories Abbott
|
|||
| Structure |
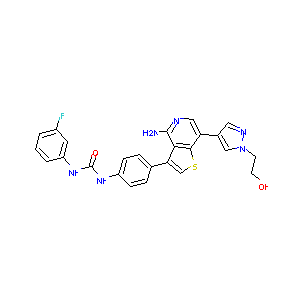 |
Download2D MOL |
||
| Formula |
C25H21FN6O2S
|
|||
| Canonical SMILES |
C1=CC(=CC(=C1)F)NC(=O)NC2=CC=C(C=C2)C3=CSC4=C3C(=NC=C4C5=CN(N=C5)CCO)N
|
|||
| InChI |
1S/C25H21FN6O2S/c26-17-2-1-3-19(10-17)31-25(34)30-18-6-4-15(5-7-18)21-14-35-23-20(12-28-24(27)22(21)23)16-11-29-32(13-16)8-9-33/h1-7,10-14,33H,8-9H2,(H2,27,28)(H2,30,31,34)
|
|||
| InChIKey |
WPHKIQPVPYJNAX-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 1227939-82-3
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT02478320) Phase II Study of Ilorasertib (ABT348) in Patients With CDKN2A Deficient Solid Tumors. | |||
| REF 2 | Preclinical characterization of ABT-348, a kinase inhibitor targeting the aurora, vascular endothelial growth factor receptor/platelet-derived growth factor receptor, and Src kinase families. J Pharmacol Exp Ther. 2012 Dec;343(3):617-27. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

