Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0O2SR
|
|||
| Former ID |
DAP001409
|
|||
| Drug Name |
Nicorandil
|
|||
| Synonyms |
Adancor; Dancor; Ikorel; Nicorandilum; Sigmart; Aventis Brand of Nicorandil; Aventis Pharma Brand of Nicorandil; Merck Brand of Nicorandil; Merck Lipha Sante Brand of Nicorandil; Nicorandil Aventis Brand; Nicorandil Merck Brand; Rhone Poulenc Rorer Brand of Nicorandil; SG 75; SG75; Ikorel (TN); Nicorandilum [INN-Latin]; RP-46417; Rhone-Poulenc Rorer Brand of Nicorandil; SG-75; Sigmart (TN); Nitrate, 2-Nicotinamidethyl; Nitrate, 2-Nicotinamidoethyl; N-(2-Hydroxyethyl)nicotinamide nitrate; Nicorandil (JP15/USAN/INN); Nicorandil [USAN:BAN:INN:JAN]; N-(2-Hydroxyethyl)nicotinamide nitrate (ester); N-[2-(Nitrooxy)ethyl]pyridine-3-carboxamide; 2 Nicotinamidethyl Nitrate; 2 Nicotinamidoethyl Nitrate; 2-(Nicotinamido)ethyl nitrat; 2-(Pyridine-3-carboxamido)ethyl Nitrate; 2-(pyridine-3-carbonylamino)ethyl nitrate; 2-Nicotinamidethyl Nitrate; 2-Nicotinamidoethyl nitrate; 2-[(pyridin-3-ylcarbonyl)amino]ethylnitrat
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Angina pectoris [ICD-11: BA40; ICD-9: 413] | Approved | [1], [2] | |
| Therapeutic Class |
Vasodilator Agents
|
|||
| Company |
Chugai; Roche; Sanofi-Aventis; Merk KGaA; Pfizer; 3M Pharmaceutical
|
|||
| Structure |
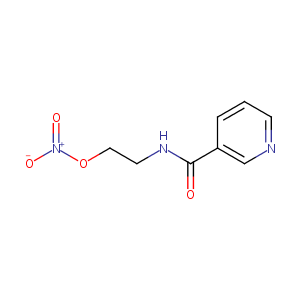 |
Download2D MOL |
||
| Formula |
C8H9N3O4
|
|||
| Canonical SMILES |
C1=CC(=CN=C1)C(=O)NCCO[N+](=O)[O-]
|
|||
| InChI |
1S/C8H9N3O4/c12-8(7-2-1-3-9-6-7)10-4-5-15-11(13)14/h1-3,6H,4-5H2,(H,10,12)
|
|||
| InChIKey |
LBHIOVVIQHSOQN-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 65141-46-0
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
586024, 1916767, 5278428, 7848872, 7980121, 8180260, 11114278, 12014252, 14797708, 26719873, 34712504, 46386683, 49681787, 49834470, 50031637, 50104982, 53788263, 57313114, 77340175, 81040873, 81093242, 85148372, 85788091, 87561371, 88477210, 92308506, 92308908, 92721812, 103198688, 103858488, 104253442, 104353163, 106136910, 115354362, 117540196, 121362729, 124659032, 124757566, 124799992, 125164370, 125355483, 125433860, 126603603, 126627520, 126657351, 126667237, 128464451, 131322571, 134338617, 135008321
|
|||
| ChEBI ID |
CHEBI:31905
|
|||
| ADReCS Drug ID | BADD_D01562 | |||
| SuperDrug ATC ID |
C01DX16
|
|||
| SuperDrug CAS ID |
cas=065141460
|
|||
| Interaction between the Drug and Microbe | Top | |||
|---|---|---|---|---|
| The Abundace of Studied Microbe(s) Regulated by Drug | ||||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Eubacteriales | ||||
|
Studied Microbe: Ruminococcus bromii
Show/Hide Hierarchy
|
[3] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experiment Method | High-throughput screening | |||
| Description | The abundance of Ruminococcus bromii was decreased by Nicorandil (adjusted p-values: 2.27E-03). | |||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | ATP-binding cassette transporter C9 (ABCC9) | Target Info | Activator | [4], [5], [6] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2411). | |||
| REF 2 | Pharmacologic therapeutics for cardiac reperfusion injury. Expert Opin Emerg Drugs. 2007 Sep;12(3):367-88. | |||
| REF 3 | Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018 Mar 29;555(7698):623-628. | |||
| REF 4 | A functional role of the C-terminal 42 amino acids of SUR2A and SUR2B in the physiology and pharmacology of cardiovascular ATP-sensitive K(+) chann... J Mol Cell Cardiol. 2005 Jul;39(1):1-6. | |||
| REF 5 | Structural basis for the interference between nicorandil and sulfonylurea action. Diabetes. 2001 Oct;50(10):2253-9. | |||
| REF 6 | The molecular basis of the specificity of action of K(ATP) channel openers. EMBO J. 2000 Dec 15;19(24):6644-51. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

