Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0H3JF
|
|||
| Former ID |
DNCL002201
|
|||
| Drug Name |
VTX-2337
|
|||
| Synonyms |
Motolimod; 926927-61-9; VTX-2337; Motolimod (VTX-2337); UNII-WP6PY72ZH3; VTX-378; VTX 2337; WP6PY72ZH3; AK171372; Motolimod [USAN:INN]; Motolimod (USAN/INN); SCHEMBL254984; GTPL9026; CHEMBL3301618; KS-00000SZB; DTXSID10239107; MolPort-039-136-930; EX-A1102; BCP16667; ZINC34946588; s7161; AKOS025289797; SB16930; DB12303; HY-13773; KB-146010; J3.657.311B; D10716; 2-amino-N,N-dipropyl-8-[4-(pyrrolidine-1-carbonyl)phenyl]-3H-1-benzazepine-4-carboxamide
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Allergy [ICD-11: 4A80-4A85; ICD-10: T78.4; ICD-9: 995.3] | Discontinued in Phase 2 | [1] | |
| Company |
VentiRx Pharmaceuticals
|
|||
| Structure |
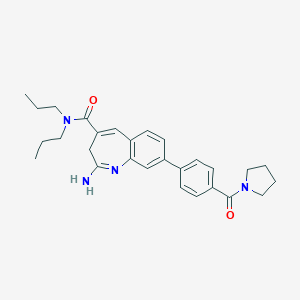 |
Download2D MOL |
||
| Formula |
C28H34N4O2
|
|||
| Canonical SMILES |
CCCN(CCC)C(=O)C1=CC2=C(C=C(C=C2)C3=CC=C(C=C3)C(=O)N4CCCC4)N=C(C1)N
|
|||
| InChI |
1S/C28H34N4O2/c1-3-13-31(14-4-2)28(34)24-17-23-12-11-22(18-25(23)30-26(29)19-24)20-7-9-21(10-8-20)27(33)32-15-5-6-16-32/h7-12,17-18H,3-6,13-16,19H2,1-2H3,(H2,29,30)
|
|||
| InChIKey |
QSPOQCXMGPDIHI-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 926927-61-9
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Toll-like receptor 8 (TLR8) | Target Info | Modulator | [2] |
| KEGG Pathway | Toll-like receptor signaling pathway | |||
| NetPath Pathway | Leptin Signaling Pathway | |||
| TCR Signaling Pathway | ||||
| Panther Pathway | Toll receptor signaling pathway | |||
| Reactome | Trafficking and processing of endosomal TLR | |||
| TRAF6 mediated IRF7 activation in TLR7/8 or 9 signaling | ||||
| TRAF6 mediated induction of NFkB and MAP kinases upon TLR7/8 or 9 activation | ||||
| MyD88 dependent cascade initiated on endosome | ||||
| WikiPathways | Toll-like receptor signaling pathway | |||
| Toll-Like Receptors Cascades | ||||
| MyD88 dependent cascade initiated on endosome | ||||
| Trafficking and processing of endosomal TLR | ||||
| Regulation of toll-like receptor signaling pathway | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT01666444) VTX-2337 and Pegylated Liposomal Doxorubicin (PLD) in Patients With Recurrent or Persistent Epithelial Ovarian, Fallopian Tube or Primary Peritoneal Cancer. U.S. National Institutes of Health. | |||
| REF 2 | Clinical pipeline report, company report or official report of Array BioPharma (Drug: Motolimod / VTX2337). | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

