Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D07QCE
|
|||
| Former ID |
DAP000782
|
|||
| Drug Name |
Azathioprine
|
|||
| Synonyms |
Azamun; Azanin; Azasan; Azathioprin; Azathioprinum; Azathiopurine; Azatioprin; Azatioprina; Azothioprine; Ccucol; Cytostatics; Immuran; Imuran; Imurek; Imurel; Methylnitroimidazolylmercaptopurine; Muran; Azamun [Czech]; Azathioprine sodium; Azatiopr in; A 4638; BW 57322; Azamun (TN); Azasan (TN); Azathioprinum [INN-Latin]; Azatioprina [INN-Spanish]; BW 57-322; Imuran (TN); Imurel (TN); [Methyl(nitroimidazolyl)mercaptopurine]; AI-981/34845012; BW-57-322; Azathioprine (JP15/USP/INN); Azathioprine [USAN:INN:BAN:JAN]; B. W. 57-322; Thiopurine 6-(1-methyl-4-nitroimidazol-5-yl); Azasan, Imuran, Azamun, BW-57-322, NSC-39084, Azathioprine; 6-((1-Methyl-4-nitro-1H-imidazol-5-yl)thio)-1H-purine; 6-((1-Methyl-4-nitroimidazol-5-yl)thio)purine; 6-(1'-Methyl-4'-nitro-5'-imidazolyl)-mercaptopurine; 6-(1'-Methyl-4'-nitro-5'-imidazolyl)mercaptopurine; 6-(1-Methyl-4-nitroimidazol-5-yl)thiopurine; 6-(1-Methyl-4-nitroimidazol-5-ylthio)purin; 6-(1-Methyl-4-nitroimidazol-5-ylthio)purin [Czech]; 6-(1-Methyl-p-nitro-5-imidazolyl)-thiopurine; 6-(1-Methyl-p-nitro-5-imidazolyl)thiopurine; 6-(3-Methyl-5-nitro-3H-imidazol-4-ylsulfanyl)-7H-purine; 6-(3-methyl-5-nitroimidazol-4-yl)sulfanyl-7H-purine; 6-(Methyl-p-nitro-5-imidazolyl)-thiopurine; 6-(Methyl-p-nitro-5-imidazolyl)thiopurine; 6-({4-nitro-1-methyl-1H-imidazol-5-yl}sulfanyl)-7H-purine; 6-1'-Methyl,4'-nitro,5'-imidazolyl mercaptopurine; 6-[(1-Methyl-4-nitroimidazol-5-yl)-thio] purine; 6-[(1-Methyl-4-nitroimidazol-5-yl)thio]purine; 6-[(1-methyl-4-nitro-1H-imidazol-5-yl)sulfanyl]-7H-purine; 6-[(1-methyl-4-nitro-1H-imidazol-5-yl)thio]-1H-Purine; 6-[(1-methyl-4-nitro-1H-imidazol-5-yl)thio]-7H-purine; 6-[(1-methyl-4-nitro-1H-imidazol-5-yl)thio]-9H-purine
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Organ transplant rejection [ICD-11: NE84; ICD-9: 279.5, 996] | Approved | [1], [2] | |
| Therapeutic Class |
Immunosuppressive Agents
|
|||
| Company |
GlaxoSmithKline
|
|||
| Structure |
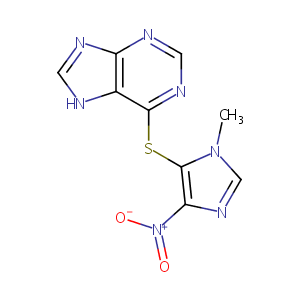 |
Download2D MOL |
||
| Formula |
C9H7N7O2S
|
|||
| Canonical SMILES |
CN1C=NC(=C1SC2=NC=NC3=C2NC=N3)[N+](=O)[O-]
|
|||
| InChI |
1S/C9H7N7O2S/c1-15-4-14-7(16(17)18)9(15)19-8-5-6(11-2-10-5)12-3-13-8/h2-4H,1H3,(H,10,11,12,13)
|
|||
| InChIKey |
LMEKQMALGUDUQG-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 446-86-6
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
9055, 545491, 4476272, 4486208, 4503338, 7592075, 7695226, 7847305, 7978746, 8149210, 8151523, 10321142, 10560483, 10584680, 11110740, 11110741, 11142069, 11335761, 11361000, 11362878, 11364839, 11365440, 11367401, 11368002, 11369963, 11371476, 11373003, 11373785, 11375563, 11376164, 11378131, 11461972, 11466122, 11467242, 11483991, 11485723, 11487884, 11490124, 11492058, 11493878, 11524329, 11533962, 11536345, 12294767, 14775167, 14824335, 15222101, 17390031, 17404591, 24278074
|
|||
| ChEBI ID |
CHEBI:2948
|
|||
| ADReCS Drug ID | BADD_D00195 ; BADD_D00196 | |||
| SuperDrug ATC ID |
L04AX01
|
|||
| SuperDrug CAS ID |
cas=000446866
|
|||
| Interaction between the Drug and Microbe | Top | |||
|---|---|---|---|---|
| The Metabolism of Drug Affected by Studied Microbe(s) | ||||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Gut microbiota | ||||
| Studied Microbe: Gut microbiota unspecific | [3] | |||
| Experimental Method | High-throughput screening | |||
| Description | Azathioprine can be metabolized by gut microbiota. | |||
| The Abundace of Studied Microbe(s) Regulated by Drug | ||||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Bacteroidales | ||||
|
Studied Microbe: Prevotella copri
Show/Hide Hierarchy
|
[4] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experiment Method | High-throughput screening | |||
| Description | The abundance of Prevotella copri was decreased by Azathioprine (adjusted p-values: 1.71E-04). | |||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Coriobacteriales | ||||
|
Studied Microbe: Collinsella aerofaciens
Show/Hide Hierarchy
|
[4] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experiment Method | High-throughput screening | |||
| Description | The abundance of Collinsella aerofaciens was decreased by Azathioprine (adjusted p-values: 3.22E-03). | |||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Eggerthellales | ||||
|
Studied Microbe: Eggerthella lenta
Show/Hide Hierarchy
|
[4] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experiment Method | High-throughput screening | |||
| Description | The abundance of Eggerthella lenta was decreased by Azathioprine (adjusted p-values: 7.57E-03). | |||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Eubacteriales | ||||
|
Studied Microbe: Blautia obeum
Show/Hide Hierarchy
|
[4] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experiment Method | High-throughput screening | |||
| Description | The abundance of Blautia obeum was decreased by Azathioprine (adjusted p-values: 9.94E-05). | |||
|
Studied Microbe: Clostridioides difficile
Show/Hide Hierarchy
|
[4] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experiment Method | High-throughput screening | |||
| Description | The abundance of Clostridioides difficile was decreased by Azathioprine (adjusted p-values: 3.13E-05). | |||
|
Studied Microbe: Coprococcus comes
Show/Hide Hierarchy
|
[4] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experiment Method | High-throughput screening | |||
| Description | The abundance of Coprococcus comes was decreased by Azathioprine (adjusted p-values: 7.36E-04). | |||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Veillonellales | ||||
|
Studied Microbe: Veillonella parvula
Show/Hide Hierarchy
|
[4] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experiment Method | High-throughput screening | |||
| Description | The abundance of Veillonella parvula was decreased by Azathioprine (adjusted p-values: 2.82E-03). | |||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Amidophosphoribosyltransferase (PPAT) | Target Info | Inhibitor | [5], [6] |
| BioCyc | Purine nucleotides degradation | |||
| Urate biosynthesis/inosine 5'-phosphate degradation | ||||
| Guanosine nucleotides de novo biosynthesis | ||||
| Superpathway of purine nucleotide salvage | ||||
| Purine nucleotides de novo biosynthesis | ||||
| Guanosine ribonucleotides de novo biosynthesis | ||||
| KEGG Pathway | Purine metabolism | |||
| Drug metabolism - other enzymes | ||||
| Metabolic pathways | ||||
| Pathwhiz Pathway | Purine Metabolism | |||
| Reactome | Purine ribonucleoside monophosphate biosynthesis | |||
| WikiPathways | Nucleotide Metabolism | |||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7120). | |||
| REF 2 | Emerging drugs for idiopathic thrombocytopenic purpura in adults. Expert Opin Emerg Drugs. 2008 Jun;13(2):237-54. | |||
| REF 3 | Personalized Mapping of Drug Metabolism by the Human Gut Microbiome. Cell. 2020 Jun 25;181(7):1661-1679.e22. | |||
| REF 4 | Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018 Mar 29;555(7698):623-628. | |||
| REF 5 | IMPDH activity in thiopurine-treated patients with inflammatory bowel disease - relation to TPMT activity and metabolite concentrations. Br J Clin Pharmacol. 2008 Jan;65(1):69-77. | |||
| REF 6 | Immunosuppressive drugs and renal transplantation. G Ital Nefrol. 2005 Nov-Dec;22 Suppl 33:S76-9. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

