Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D04XIO
|
|||
| Former ID |
DIB013859
|
|||
| Drug Name |
Dutogliptin
|
|||
| Synonyms |
Dutogliptin tartrate; PHX-1004; PHX-1149; PHX-1149T; DPP IV inhibitors (diabetes), Phenomix; DPP IV program (diabetes), Phenomix
Click to Show/Hide
|
|||
| Indication | Type-2 diabetes [ICD-11: 5A11; ICD-9: 250] | Phase 3 | [1] | |
| Company |
Phenomix Corp
|
|||
| Structure |
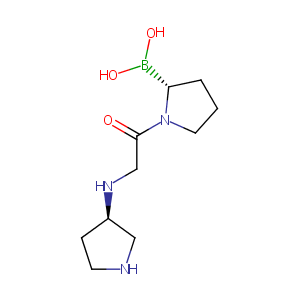 |
Download2D MOL |
||
| Formula |
C10H20BN3O3
|
|||
| Canonical SMILES |
B(C1CCCN1C(=O)CNC2CCNC2)(O)O
|
|||
| InChI |
1S/C10H20BN3O3/c15-10(7-13-8-3-4-12-6-8)14-5-1-2-9(14)11(16)17/h8-9,12-13,16-17H,1-7H2/t8-,9+/m1/s1
|
|||
| InChIKey |
DVJAMEIQRSHVKC-BDAKNGLRSA-N
|
|||
| CAS Number |
CAS 852329-66-9
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Dipeptidyl peptidase 4 (DPP-4) | Target Info | Inhibitor | [2] |
| KEGG Pathway | Protein digestion and absorption | |||
| NetPath Pathway | IL2 Signaling Pathway | |||
| TGF_beta_Receptor Signaling Pathway | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT00690638) Safety and Efficacy Study of Dutogliptin/PHX1149T to Treat Type 2 Diabetes Mellitus. U.S. National Institutes of Health. | |||
| REF 2 | Dutogliptin, a selective DPP4 inhibitor, improves glycaemic control in patients with type 2 diabetes: a 12-week, double-blind, randomized, placebo-controlled, multicentre trial. Diabetes Obes Metab. 2010 Apr;12(4):348-55. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

